1 はじめに 38
1.1 調査目的 38
1.2 市場の定義 38
1.3 調査範囲 39
1.3.1 対象市場と地域範囲 39
1.3.2 対象範囲と除外範囲 40
1.3.3 考慮した年数 41
1.4 考慮した通貨 41
1.5 利害関係者 41
1.6 市場との調整 41
2 調査方法 42
2.1 調査データ 42
2.1.1 二次データ 43
2.1.1.1 二次ソースからの主要データ 44
2.1.2 一次データ 44
2.1.2.1 一次情報源 45
2.1.2.2 主要な業界インサイト 46
2.1.2.3 一次情報源からの主要データ 46
2.1.2.4 一次インタビューの内訳 47
2.2 市場規模の推定 48
2.2.1 ボトムアップアプローチ 48
2.2.1.1 アプローチ1:企業収益推定アプローチ 48
2.2.1.2 アプローチ2:企業プレゼンテーションと一次ヒアリング 49
2.2.1.3 アプローチ3:プライマリーインタビュー 49
2.2.1.4 成長予測 49
2.2.1.5 CAGR予測 49
2.2.2 トップダウンアプローチ 50
2.3 市場の内訳とデータの三角測量 51
2.4 調査の前提 52
2.4.1 調査に関する前提条件 52
2.4.2 パラメトリックな前提 52
2.5 調査の限界
2.6 リスク評価
3 エグゼクティブ・サマリー 54
4 プレミアムインサイト 59
4.1 IVD試薬市場の概要 59
4.2 アジア太平洋地域:IVD試薬市場:国別 60
4.3 北米:IVD試薬市場:検査タイプ別、国別 60
4.4 IVD試薬市場:国別 61
5 市場の概要 62
5.1 はじめに 62
5.2 市場ダイナミクス 62
5.2.1 推進要因 63
5.2.1.1 高齢者人口の増加 63
5.2.1.2 ポイントオブケア検査と自動分析装置への漸進的シフト 64
5.2.1.3 早期診断と個別化医療の増加 65
5.2.1.4 第三者機関による品質管理の採用増加 66
5.2.1.5 バイオテクノロジーとバイオ医薬品産業の成長 67
5.2.1.6 研究開発活動のための資金調達の増加 67
5.2.2 阻害要因 68
5.2.2.1 不利な償還シナリオ 68
5.2.2.2 厳しい規制要件 68
5.2.3 機会 69
5.2.3.1 バイオマーカー開発の増加 69
5.2.3.2 コンパニオン診断薬の重要性の高まり 70
5.2.3.3 新興国における市場成長機会 71
5.2.3.4 デジタル化の進展 72
5.2.4 課題 73
5.2.4.1 臨床プロセスにおける運用上の課題 73
5.3 顧客のビジネスに影響を与えるトレンド/混乱 74
5.4 価格分析 75
5.4.1 主要企業の平均販売価格動向(用途別) 75
5.4.2 主要企業の平均販売価格動向(地域別) 76
5.4.3 技術別の平均販売価格動向 77
5.5 バリューチェーン分析 77
5.6 サプライチェーン分析 79
5.7 エコシステム分析 80
5.8 投資と資金調達のシナリオ 81
5.9 技術分析 81
5.9.1 主要技術 82
5.9.2 隣接技術 83
5.9.2.1 多重化ポイントオブケア検査 83
5.9.2.2 核酸ラテラルフロー免疫測定法 83
5.9.2.3 DNAベースの検出 83
5.9.2.4 免疫診断法 83
5.9.2.5 デュアルパス技術 83
5.10 特許分析 84
5.11 貿易分析 85
5.11.1 輸入データ 85
5.11.2 輸出データ 85
5.12 主要会議・イベント(2024-2025年) 86
5.13 ポーターのファイブフォース分析 86
5.13.1 新規参入の脅威 87
5.13.2 代替品の脅威 87
5.13.3 供給者の交渉力 88
5.13.4 買い手の交渉力 88
5.13.5 競合の激しさ 88
5.14 規制分析 88
5.14.1 規制の状況 88
5.14.1.1 北米 89
5.14.1.1.1 米国 89
5.14.1.1.2 カナダ 90
5.14.1.2 欧州 90
5.14.1.3 アジア太平洋 92
5.14.1.3.1 中国 92
5.14.1.3.2 日本 92
5.14.1.3.3 インド 93
5.14.1.4 中南米 93
5.14.1.4.1 ブラジル 93
5.14.1.5 中東 94
5.14.2 規制機関、政府機関、その他の組織 94
5.14.2.1 北米 94
5.14.2.2 欧州 95
5.14.2.3 アジア太平洋 95
5.14.2.4 ラテンアメリカ 96
5.14.2.5 中東・アフリカ 96
5.14.2.6 GCC諸国 96
5.15 主要ステークホルダーと購買基準 97
5.15.1 購入プロセスにおける主要ステークホルダー 97
5.15.2 購入基準 98
5.16 AI/ジェネレーティブAIがIVD試薬市場に与える影響 98
5.16.1 導入 98
5.16.2 IVD試薬の市場ポテンシャル 98
5.16.3 AIの使用事例 99
5.16.4 AIを導入する主要企業 100
5.16.5 IVD試薬におけるジェネレーティブAIの将来性 100
6 IVD試薬市場、タイプ別 101
6.1 導入 102
6.2 抗体 102
6.2.1 モノクローナル抗体 105
6.2.1.1 癌罹患率の増加が成長を刺激 105
6.2.2 ポリクローナル抗体 107
6.2.2.1 感染症や自己免疫疾患の有病率の増加が成長を後押し 107
成長を支える 107
6.3 抗原、精製タンパク質、ペプチド 110
6.3.1 高度な精密医療への注目の高まりが成長を促進 110
成長を促す 110
6.4 オリゴヌクレオチド 113
6.4.1 分子診断への志向の高まりが市場を牽引 113
6.5 核酸プローブ 116
6.5.1 病気の早期発見と低存在バイオマーカーのモニタリングの必要性が成長を後押し 116
6.6 その他のIVD試薬 119
7 IVD試薬市場:技術別 123
7.1 導入 124
7.2 イムノアッセイ 124
7.2.1 自動化傾向の高まりが成長を促進 124
7.3 臨床化学 128
7.3.1 糖尿病有病率の上昇がセグメントを押し上げる 128
7.4 分子診断薬 131
7.4.1 血液スクリーニングとポイントオブケア検査の需要拡大が
市場が活性化 131
7.5 血液学 133
7.5.1 幹細胞研究への注目の高まりが成長を加速 133
7.6 微生物学 136
7.6.1 微生物感染症の流行増加が市場を刺激 136
7.7 凝固・止血 138
7.7.1 抗凝固療法の普及が市場を牽引 138
7.8 尿検査 141
7.8.1 腎臓疾患の有病率の上昇が市場成長を支える 141
7.9 クロマトグラフィー&質量分析 144
7.9.1 疾患スクリーニング、法医学分析、薬物治療における用途の増加、
薬物療法が市場を押し上げる 144
7.10 免疫組織化学 146
7.10.1 がん診断のための臨床検査数の増加が市場を牽引 146
市場を牽引 146
8 IVD試薬市場(用途別) 150
8.1 導入 151
8.2 感染症 151
8.2.1 製品の上市と承認の増加が市場を押し上げる 151
8.3 がん領域への応用 154
8.3.1 早期診断と質の高い治療が重視されるようになり
市場が活性化 154
8.4 内分泌アプリケーション 157
8.4.1 増加する糖尿病と甲状腺関連疾患
が成長を維持する 157
8.5 心臓病アプリケーション 160
8.5.1 先進国および発展途上国におけるライフスタイルの変化が
が市場を牽引 160
8.6 血液スクリーニング 163
8.6.1 自動診断機器の採用が増加。
成長を促進 163
8.7 遺伝子検査 166
8.7.1 希少疾患や致死的疾患の診断に遺伝子検査が採用されるケースが増加し
成長が持続 166
8.8 自己免疫疾患 169
8.8.1 臨床検査技術、試薬、代替アッセイ手法の進歩が成長、
および代替アッセイ手法の進歩が市場を牽引 169
8.9 アレルギー診断 172
8.9.1 アレルギー治療と予防への注目の高まり
成長を加速 172
8.10 薬物モニタリング・検査 175
8.10.1 乱用薬物検査の実施拡大が市場を牽引 175
8.11 その他の用途 178
9 IVD試薬市場:検査タイプ別 182
9.1 導入 183
9.2 ラボ検査 183
9.2.1 自動化ニーズの高まりが成長を促進 183
9.3 ポイントオブケア検査 186
9.3.1 患者の状態を詳細にモニタリングする必要性が成長を促進 186
10 IVD試薬市場:エンドユーザー別 190
10.1 導入 191
10.2 病院・診療所 191
10.2.1 専門的な診断検査の増加が成長を加速 191
10.3 臨床検査室 194
10.3.1 大規模基準検査室 198
10.3.1.1 専門的な検査能力と最小のターンアラウンドタイムに対するニーズが
市場を押し上げる 198
10.3.2 中小規模の検査室 201
10.3.2.1 自動化・半自動化機器の採用拡大が成長を支える 201
成長を支える 201
10.4 血液銀行 203
10.4.1 外傷症例の増加と高度な外科手術の可能性が市場を後押し 203
10.5 在宅介護環境 206
10.5.1 在宅検査キットへの嗜好の高まりが市場を牽引 206
10.6 製薬・バイオテクノロジー企業 209
10.6.1 研究開発努力の増加が市場の成長に寄与
成長への寄与 209
10.7 学術機関 212
10.7.1 産学連携の増加が成長を促進 212
10.8 その他のエンドユーザー 216
11 IVD試薬市場:地域別 219
11.1 はじめに 220
11.2 北米 220
11.2.1 北米のマクロ経済見通し 221
11.2.2 米国 226
11.2.2.1 確立された償還の枠組みが市場を牽引 226
11.2.3 カナダ 230
11.2.3.1 慢性疾患および感染性生活習慣病の罹患率の増加が市場を牽引 230
が市場を牽引 230
11.3 欧州 235
11.3.1 欧州のマクロ経済見通し 236
11.3.2 ドイツ 240
11.3.2.1 臨床診断研究への投資の増加が市場を牽引 240
11.3.3 英国 244
11.3.3.1 ゲノムベースの検査導入の増加が成長を促進 244
11.3.4 フランス 248
11.3.4.1 高い医療費とゲノム医療への投資の増加が市場を押し上げる 248
市場を押し上げる 248
11.3.5 イタリア 251
11.3.5.1 老年人口の増加とそれに伴う慢性疾患の増加が市場成長を支える 251
市場成長を支える 251
11.3.6 スペイン 255
11.3.6.1 免疫測定試薬の採用増加が市場を牽引 255
11.3.7 その他の欧州 258
11.4 アジア太平洋地域 263
11.4.1 アジア太平洋地域のマクロ経済見通し 263
11.4.2 日本 268
11.4.2.1 社会医療保険制度の増加が成長を促進 268
11.4.3 中国 272
11.4.3.1 予防医療への関心の高まりが需要を後押し 272
11.4.4 インド 276
11.4.4.1 慢性疾患の蔓延が市場を刺激 276
11.4.5 その他のアジア太平洋地域 280
11.5 ラテンアメリカ 284
11.5.1 ラテンアメリカのマクロ経済見通し 284
11.5.2 ブラジル 288
11.5.2.1 成長を刺激する糖尿病有病率の上昇 288
11.5.3 メキシコ 291
11.5.3.1 臨床検査施設設立の増加が市場を押し上げる 291
11.5.4 その他のラテンアメリカ 295
11.6 中東・アフリカ 298
11.6.1 免疫学、出生前検査、がん検査への関心の高まりが市場を牽引 298
市場を牽引 298
11.6.2 中東・アフリカのマクロ経済見通し 299
11.7 GCC諸国 303
11.7.1 GCC諸国のマクロ経済見通し 303
11.7.2 サウジアラビア 307
11.7.2.1 成長促進に向けた医療支出の増加 307
11.7.3 アラブ首長国連邦 310
11.7.3.1 成長を促進する高度医療インフラ整備の増加
成長を促進 310
11.7.4 その他のGCC諸国 314
12 競争環境 318
12.1 概要 318
12.2 主要プレーヤーの戦略/勝利への権利(2023年) 318
12.2.1 IVD試薬市場で主要企業が採用した戦略の概要(2021~2023年) 318
市場(2021-2023年) 318
12.3 収益分析、2021-2023年 319
12.4 市場シェア分析、2023年 320
12.5 企業評価と財務指標 322
12.6 ブランド/製品の比較 324
12.7 企業評価マトリックス:主要企業、2023年 325
12.7.1 スター企業 325
12.7.2 新興リーダー 325
12.7.3 浸透型プレーヤー 325
12.7.4 参加企業 325
12.7.5 企業フットプリント:主要プレーヤー、2023年 327
12.7.5.1 企業フットプリント 327
12.7.5.2 技術のフットプリント 328
12.7.5.3 アプリケーションフットプリント 329
12.7.5.4 検査タイプのフットプリント 330
12.7.5.5 地域別フットプリント 331
12.8 企業評価マトリクス:新興企業/SM(2023年) 332
12.8.1 進歩的企業 332
12.8.2 対応力のある企業 332
12.8.3 ダイナミックな企業 332
12.8.4 スタートアップ・ブロック 332
12.8.5 競争ベンチマーキング:新興企業/中小企業(2023年) 334
12.8.5.1 主要新興企業/中小企業の詳細リスト 334
334 12.8.5.2 主要新興企業/中小企業の競争ベンチマーク 334
12.9 競争シナリオ 335
12.9.1 製品の上市と承認 335
12.9.2 取引 336
12.9.3 事業拡大 337
337 12.9.4 その他の開発 337
13 会社プロファイル 338
13.1 主要企業 338
Danaher Corporation (US)
F. Hoffmann-La Roche Ltd (Switzerland)
Abbott (US)
Siemens Healthineers AG (Germany)
Thermo Fisher Scientific Inc. (US)
Illumina Inc. (US)
BioMerieux (France)
BD (US)
Hologic Inc. (US)
Bio-Rad Laboratories Inc. (US)
Sysmex Corporation (Japan)
QIAGEN N.V. (Netherlands)
Agilent Technologies Inc. (US)
Revvity (US)
DiaSorin S.p.A (Italy)
Grifols S.A. (Spain)
Werfen S.A. (Spain)
QuidelOrtho Corporation (US)
Chembio Diagnostics Inc. (US)
Surmodics Inc. (US)
Merck KGaA (Germany)
MEDICAL & BIOLOGICAL LABORATORIES CO.LTD. (Tokyo)
Canvax (Spain)
Prestige Diagnostics (US)
Adaltis S.r.l. (Italy)
and Randox Laboratories Ltd. (UK)
14 付録 415
14.1 ディスカッションガイド 415
14.2 Knowledgestore: マーケットサ ンドマーケッツの購読ポータル 420
14.3 カスタマイズオプション 422
14.4 関連レポート 422
14.5 著者の詳細 423
The increasing adoption of IVD technologies has led to a growing need of IVD reagents. The demand for IVD reagents is therefore expected to increase as new diagnostic technologies are getting FDA, EMA approvals from regulatory bodies.
“The oligonucleotide segment is projected to witness the highest growth rate in the IVD reagents market, by type, during the forecast period.”
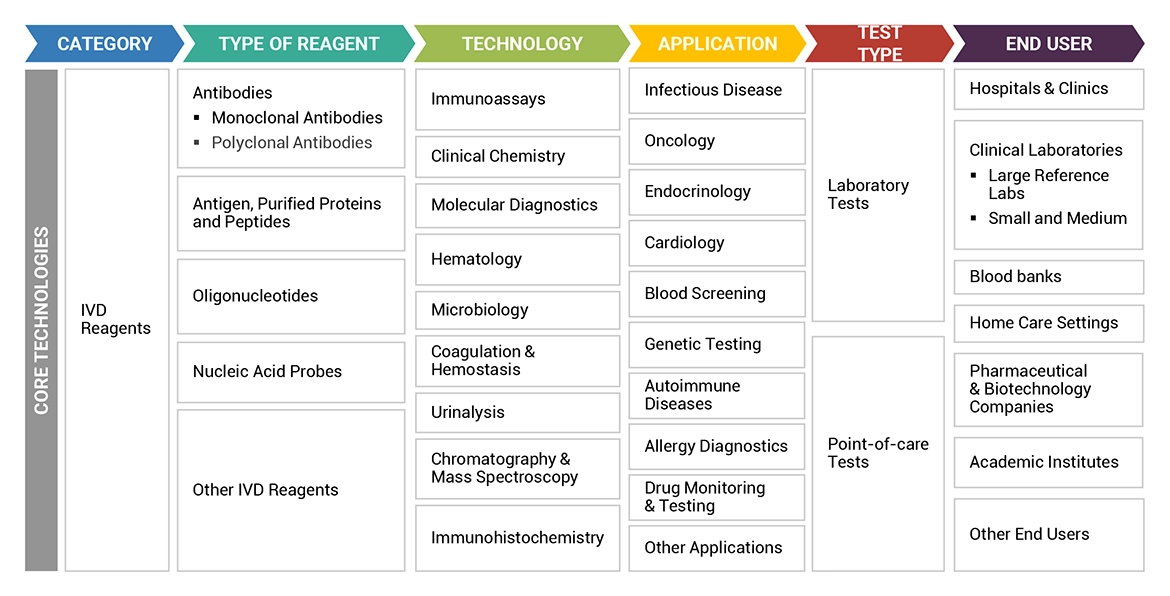
Based on type, the IVD reagents market is segmented into antibodies, antigen, purified proteins, and peptides, oligonucleotides, nucleic acid probes, and other IVD reagents. The growing adoption of PCR-based tests in diagnostics has boosted the demand for oligonucleotide in the IVD reagents market. Oligonucleotides have wide range of applications in infectious diseases and genetic testing.
“The infectious disease segment to witness the highest share in the IVD reagents market, in 2023, by application”
Based on application, the IVD reagents market is segmented into infectious diseases, oncological applications, endocrinological applications, cardiological applications, blood screening, genetic testing, autoimmune diseases, allergy diagnostics, drug monitoring & testing and other applications. The infectious diseases segment experience highest market share of IVD reagents market due to rising number of R&D activities and product approvals for infectious disease applications.
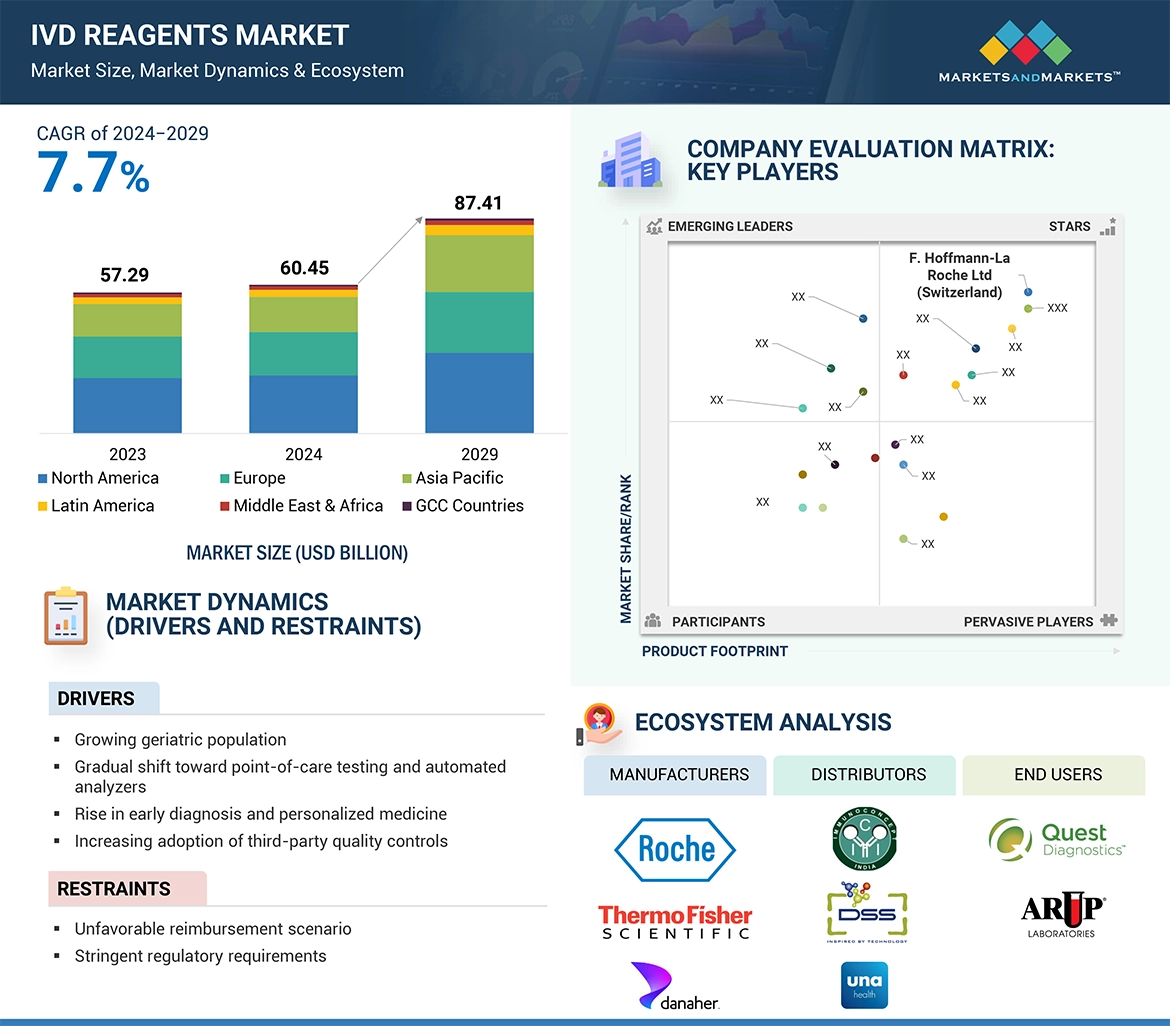
“The Asia Pacific region is projected to witness highest growth rate in the IVD reagents market during the forecast period”
The global IVD reagents market is segmented into six regions - North America, Europe, Asia Pacific, the Middle East & Africa, Latin America, and the GCC Countries. Over the span of the forecast period, the IVD reagents market is expected to grow at the fastest rate in the Asia Pacific region. Over the projected period, the Asia Pacific market is expected to develop at the greatest rate due to factors such increasing healthcare spending and an extensive population base.
The primary interviews conducted for this report can be categorized as follows:
• By Company Type: Tier 1 - 42%, Tier 2 - 30%, and Tier 3 - 28%
• By Designation: C-level - 46%, Director-level - 23%, and Others - 31%
• By Region: North America – 23%, Europe – 44%, Asia Pacific – 28%, Latin America – 3%, Middle East & Africa – 1%, and the GCC Countries – 1%
Lists of Companies Profiled in the Report:
Danaher Corporation (US), F. Hoffmann-La Roche Ltd (Switzerland), Abbott (US), Siemens Healthineers AG (Germany), Thermo Fisher Scientific Inc. (US), Illumina, Inc. (US), BioMerieux (France), BD (US), Hologic, Inc. (US), Bio-Rad Laboratories, Inc. (US), Sysmex Corporation (Japan), QIAGEN N.V. (Netherlands), Agilent Technologies, Inc. (US), Revvity (US), DiaSorin S.p.A (Italy), Grifols, S.A. (Spain), Werfen, S.A. (Spain), QuidelOrtho Corporation (US), Chembio Diagnostics, Inc. (US), Surmodics, Inc. (US), Merck KGaA (Germany), MEDICAL & BIOLOGICAL LABORATORIES CO., LTD. (Tokyo), Canvax (Spain), Prestige Diagnostics (US), Adaltis S.r.l. (Italy), and Randox Laboratories Ltd. (UK)
Study Coverage:
In this report, the IVD reagents market has been categorized based on type (antibodies, antigen, purified proteins, and peptides, oligonucleotides, nucleic acid probes, and other IVD reagents), technology (immunoassays, clinical chemistry, molecular diagnostics, hematology, microbiology, coagulation & hemostasis, urinalysis, chromatography & mass spectrometry, immunohistochemistry), application (infectious diseases, oncological applications, endocrinological application, cardiology application, blood screening, genetic testing, autoimmune diseases, allergy diagnostics, drug monitoring & testing, and other applications), test type (laboratory test, and point-of-care tests), end user (hospitals & clinics, clinical laboratories, blood banks, home care settings, pharmaceutical & biotechnology companies, academic institutes, and other end users), and region (North America, Europe, Asia Pacific, Latin America, Middle East & Africa, and the GCC Countries).
Information regarding the main drivers, restraints, opportunities, and challenges influencing the IVD reagents market's expansion is includes detail in this study. An intensive study of the key players in the IVD reagents market has been done to provide insights into their business profile, service offered, note worthy strategies, acquisitions and expansions, and other deals made pertaining to the market. This study examines the fragmented landscape of emerging IVD reagents startups.
Reasons to buy this report:
The report will help the market leaders/new entrants in this market with information on the closest approximations of the revenue numbers for the overall IVD reagents marketand the subsegments. This report will help stakeholders understand the competitive landscape and gain more insights to position their businesses better and to plan suitable go-to-market strategies. The report also helps stakeholders understand the pulse of the market and provides them with information on key market drivers, challenges, opportunities, and restaints.
The report provides insights on the following pointers:
• Analysis of key drivers: (growing geriatric population, gradual shift towards point-of-care testing and automated analyzers, rise in early diagnosis and personalized medicine, increasing adoption of third-party quality controls, growing biotechnology and biopharmaceutical industries, increasing funding for R&D activities), restraints (unfavorable reimbursement scenario, stringent regulatory requirements), opportunities (increasing development of biomarkers, rising significance of companion diagnostics, market growth opportunities in emerging economies, and growing trend of digitalization), and challenges (operational challenges in clinical process) influencing the growth of the in IVD reagents market.
• Market Development: Comprehensive information about lucrative markets – the report analyses the IVD reagents market across varied regions.
• Market Diversification: Exhaustive information about untapped geographies, and investments in the IVD reagents market
• Competitive Assessment: In-depth assessment of market shares, growth strategies, and product offerings of leading players like include F. Hoffmann-La Roche Ltd (Switzerland), Abbott (US), Danaher Corporation (US), Siemens Healthineers AG (Germany), and Thermo Fisher Scientific Inc. (US) are among others, in the IVD reagents market strategies.
1 INTRODUCTION 38
1.1 STUDY OBJECTIVES 38
1.2 MARKET DEFINITION 38
1.3 STUDY SCOPE 39
1.3.1 MARKETS COVERED AND REGIONAL SCOPE 39
1.3.2 INCLUSIONS AND EXCLUSIONS 40
1.3.3 YEARS CONSIDERED 41
1.4 CURRENCY CONSIDERED 41
1.5 STAKEHOLDERS 41
1.6 MARKET RECONCILIATION 41
2 RESEARCH METHODOLOGY 42
2.1 RESEARCH DATA 42
2.1.1 SECONDARY DATA 43
2.1.1.1 Key data from secondary sources 44
2.1.2 PRIMARY DATA 44
2.1.2.1 Primary sources 45
2.1.2.2 Key industry insights 46
2.1.2.3 Key data from primary sources 46
2.1.2.4 Breakdown of primary interviews 47
2.2 MARKET SIZE ESTIMATION 48
2.2.1 BOTTOM-UP APPROACH 48
2.2.1.1 Approach 1: Company revenue estimation approach 48
2.2.1.2 Approach 2: Presentations of companies and primary interviews 49
2.2.1.3 Approach 3: Primary interviews 49
2.2.1.4 Growth forecast 49
2.2.1.5 CAGR projections 49
2.2.2 TOP-DOWN APPROACH 50
2.3 MARKET BREAKDOWN AND DATA TRIANGULATION 51
2.4 RESEARCH ASSUMPTIONS 52
2.4.1 STUDY-RELATED ASSUMPTIONS 52
2.4.2 PARAMETRIC ASSUMPTIONS 52
2.5 RESEARCH LIMITATIONS 53
2.6 RISK ASSESSMENT 53
3 EXECUTIVE SUMMARY 54
4 PREMIUM INSIGHTS 59
4.1 IVD REAGENTS MARKET OVERVIEW 59
4.2 ASIA PACIFIC: IVD REAGENTS MARKET, BY COUNTRY 60
4.3 NORTH AMERICA: IVD REAGENTS MARKET, BY TEST TYPE AND COUNTRY 60
4.4 IVD REAGENTS MARKET, BY COUNTRY 61
5 MARKET OVERVIEW 62
5.1 INTRODUCTION 62
5.2 MARKET DYNAMICS 62
5.2.1 DRIVERS 63
5.2.1.1 Growing geriatric population 63
5.2.1.2 Gradual shift toward point-of-care testing and automated analyzers 64
5.2.1.3 Rise in early diagnosis and personalized medicine 65
5.2.1.4 Increasing adoption of third-party quality controls 66
5.2.1.5 Growing biotechnology and biopharmaceutical industries 67
5.2.1.6 Increasing funding for R&D activities 67
5.2.2 RESTRAINTS 68
5.2.2.1 Unfavorable reimbursement scenario 68
5.2.2.2 Stringent regulatory requirements 68
5.2.3 OPPORTUNITIES 69
5.2.3.1 Increasing development of biomarkers 69
5.2.3.2 Rising significance of companion diagnostics 70
5.2.3.3 Market growth opportunities in emerging economies 71
5.2.3.4 Growing trend of digitalization 72
5.2.4 CHALLENGES 73
5.2.4.1 Operational challenges in clinical process 73
5.3 TRENDS/DISRUPTIONS IMPACTING CUSTOMERS’ BUSINESSES 74
5.4 PRICING ANALYSIS 75
5.4.1 AVERAGE SELLING PRICE TREND OF KEY PLAYERS, BY APPLICATION 75
5.4.2 AVERAGE SELLING PRICE TREND OF KEY PLAYERS, BY REGION 76
5.4.3 AVERAGE SELLING PRICE TREND, BY TECHNOLOGY 77
5.5 VALUE CHAIN ANALYSIS 77
5.6 SUPPLY CHAIN ANALYSIS 79
5.7 ECOSYSTEM ANALYSIS 80
5.8 INVESTMENT AND FUNDING SCENARIO 81
5.9 TECHNOLOGY ANALYSIS 81
5.9.1 KEY TECHNOLOGIES 82
5.9.2 ADJACENT TECHNOLOGIES 83
5.9.2.1 Multiplexed point-of-care testing 83
5.9.2.2 Nucleic acid lateral flow immunoassay 83
5.9.2.3 DNA-based detection 83
5.9.2.4 Immunodiagnostics 83
5.9.2.5 Dual path technology 83
5.10 PATENT ANALYSIS 84
5.11 TRADE ANALYSIS 85
5.11.1 IMPORT DATA 85
5.11.2 EXPORT DATA 85
5.12 KEY CONFERENCES AND EVENTS, 2024−2025 86
5.13 PORTER’S FIVE FORCES ANALYSIS 86
5.13.1 THREAT OF NEW ENTRANTS 87
5.13.2 THREAT OF SUBSTITUTES 87
5.13.3 BARGAINING POWER OF SUPPLIERS 88
5.13.4 BARGAINING POWER OF BUYERS 88
5.13.5 INTENSITY OF COMPETITIVE RIVALRY 88
5.14 REGULATORY ANALYSIS 88
5.14.1 REGULATORY LANDSCAPE 88
5.14.1.1 North America 89
5.14.1.1.1 US 89
5.14.1.1.2 Canada 90
5.14.1.2 Europe 90
5.14.1.3 Asia Pacific 92
5.14.1.3.1 China 92
5.14.1.3.2 Japan 92
5.14.1.3.3 India 93
5.14.1.4 Latin America 93
5.14.1.4.1 Brazil 93
5.14.1.5 Middle East 94
5.14.2 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS 94
5.14.2.1 North America 94
5.14.2.2 Europe 95
5.14.2.3 Asia Pacific 95
5.14.2.4 Latin America 96
5.14.2.5 Middle East & Africa 96
5.14.2.6 GCC Countries 96
5.15 KEY STAKEHOLDERS AND BUYING CRITERIA 97
5.15.1 KEY STAKEHOLDERS IN BUYING PROCESS 97
5.15.2 BUYING CRITERIA 98
5.16 IMPACT OF AI/GENERATIVE AI ON IVD REAGENTS MARKET 98
5.16.1 INTRODUCTION 98
5.16.2 MARKET POTENTIAL FOR IVD REAGENTS 98
5.16.3 AI USE CASES 99
5.16.4 KEY COMPANIES IMPLEMENTING AI 100
5.16.5 FUTURE OF GENERATIVE AI ON IVD REAGENTS 100
6 IVD REAGENTS MARKET, BY TYPE 101
6.1 INTRODUCTION 102
6.2 ANTIBODIES 102
6.2.1 MONOCLONAL ANTIBODIES 105
6.2.1.1 Increasing incidence of cancer to stimulate growth 105
6.2.2 POLYCLONAL ANTIBODIES 107
6.2.2.1 Rising prevalence of infectious diseases and autoimmune disorders
to support growth 107
6.3 ANTIGENS, PURIFIED PROTEINS, AND PEPTIDES 110
6.3.1 INCREASING FOCUS ON ADVANCED PRECISION MEDICINE
TO ENCOURAGE GROWTH 110
6.4 OLIGONUCLEOTIDES 113
6.4.1 GROWING INCLINATION TOWARD MOLECULAR DIAGNOSTICS TO DRIVE MARKET 113
6.5 NUCLEIC ACID PROBES 116
6.5.1 NEED FOR EARLY DISEASE DETECTION AND MONITORING LOW-ABUNDANCE BIOMARKERS TO AID GROWTH 116
6.6 OTHER IVD REAGENTS 119
7 IVD REAGENTS MARKET, BY TECHNOLOGY 123
7.1 INTRODUCTION 124
7.2 IMMUNOASSAYS 124
7.2.1 RISING TREND OF AUTOMATION TO PROMOTE GROWTH 124
7.3 CLINICAL CHEMISTRY 128
7.3.1 RISING PREVALENCE OF DIABETES TO BOOST SEGMENT 128
7.4 MOLECULAR DIAGNOSTICS 131
7.4.1 GROWING DEMAND FOR BLOOD SCREENING AND POINT-OF-CARE TESTING
TO FUEL MARKET 131
7.5 HEMATOLOGY 133
7.5.1 INCREASING FOCUS ON STEM CELL RESEARCH TO SPEED UP GROWTH 133
7.6 MICROBIOLOGY 136
7.6.1 RISING PREVALENCE OF MICROBIAL INFECTIONS TO FUEL MARKET 136
7.7 COAGULATION & HEMOSTASIS 138
7.7.1 GROWING USE OF ANTICOAGULATION THERAPY TO PROPEL MARKET 138
7.8 URINALYSIS 141
7.8.1 RISING PREVALENCE OF KIDNEY DISEASES TO SUPPORT MARKET GROWTH 141
7.9 CHROMATOGRAPHY & MASS SPECTROMETRY 144
7.9.1 INCREASING APPLICATIONS IN DISEASE SCREENING, FORENSIC ANALYSIS,
AND DRUG THERAPY TO BOOST MARKET 144
7.10 IMMUNOHISTOCHEMISTRY 146
7.10.1 GROWING NUMBER OF LABORATORY TESTS FOR CANCER DIAGNOSIS
TO DRIVE MARKET 146
8 IVD REAGENTS MARKET, BY APPLICATION 150
8.1 INTRODUCTION 151
8.2 INFECTIOUS DISEASES 151
8.2.1 INCREASING PRODUCT LAUNCHES AND APPROVALS TO BOOST MARKET 151
8.3 ONCOLOGICAL APPLICATIONS 154
8.3.1 GROWING EMPHASIS ON EARLY DIAGNOSIS AND QUALITY TREATMENT
TO FUEL MARKET 154
8.4 ENDOCRINOLOGICAL APPLICATIONS 157
8.4.1 RISING PREVALENCE OF DIABETES AND THYROID-RELATED DISORDERS
TO SUSTAIN GROWTH 157
8.5 CARDIOLOGICAL APPLICATIONS 160
8.5.1 CHANGING LIFESTYLES IN DEVELOPED AND DEVELOPING COUNTRIES
TO PROPEL MARKET 160
8.6 BLOOD SCREENING 163
8.6.1 RISING ADOPTION OF AUTOMATED DIAGNOSTIC INSTRUMENTS
TO FACILITATE GROWTH 163
8.7 GENETIC TESTING 166
8.7.1 INCREASING ADOPTION OF GENETIC TESTING TO DIAGNOSE RARE
AND FATAL DISEASES TO SUSTAIN GROWTH 166
8.8 AUTOIMMUNE DISEASES 169
8.8.1 GROWING ADVANCEMENTS IN LABORATORY TECHNOLOGY, REAGENTS,
AND ALTERNATIVE ASSAY METHODOLOGIES TO DRIVE MARKET 169
8.9 ALLERGY DIAGNOSTICS 172
8.9.1 GROWING FOCUS ON ALLERGY THERAPY AND PREVENTION
TO ACCELERATE GROWTH 172
8.10 DRUG MONITORING & TESTING 175
8.10.1 GROWING IMPLEMENTATION OF DRUGS-OF-ABUSE TESTING TO DRIVE MARKET 175
8.11 OTHER APPLICATIONS 178
9 IVD REAGENTS MARKET, BY TEST TYPE 182
9.1 INTRODUCTION 183
9.2 LABORATORY TESTS 183
9.2.1 INCREASING NEED FOR AUTOMATION TO EXPEDITE GROWTH 183
9.3 POINT-OF-CARE TESTS 186
9.3.1 NEED TO CLOSELY MONITOR PATIENT CONDITIONS TO AUGMENT GROWTH 186
10 IVD REAGENTS MARKET, BY END USER 190
10.1 INTRODUCTION 191
10.2 HOSPITALS & CLINICS 191
10.2.1 INCREASING SPECIALTY DIAGNOSTIC TESTS TO ACCELERATE GROWTH 191
10.3 CLINICAL LABORATORIES 194
10.3.1 LARGE REFERENCE LABORATORIES 198
10.3.1.1 Need for specialized testing capabilities and minimal turnaround
time to boost market 198
10.3.2 SMALL & MEDIUM-SIZED LABORATORIES 201
10.3.2.1 Growing adoption of automated and semi-automated instruments
to support growth 201
10.4 BLOOD BANKS 203
10.4.1 GROWING NUMBER OF TRAUMA CASES AND AVAILABILITY OF SOPHISTICATED SURGICAL PROCEDURES TO FUEL MARKET 203
10.5 HOME CARE SETTINGS 206
10.5.1 GROWING PREFERENCE FOR AT-HOME TESTING KITS TO DRIVE MARKET 206
10.6 PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES 209
10.6.1 INCREASING RESEARCH & DEVELOPMENT EFFORTS TO CONTRIBUTE
TO GROWTH 209
10.7 ACADEMIC INSTITUTES 212
10.7.1 INCREASING INDUSTRY-ACADEMIA COLLABORATIONS TO FAVOR GROWTH 212
10.8 OTHER END USERS 216
11 IVD REAGENTS MARKET, BY REGION 219
11.1 INTRODUCTION 220
11.2 NORTH AMERICA 220
11.2.1 MACROECONOMIC OUTLOOK FOR NORTH AMERICA 221
11.2.2 US 226
11.2.2.1 Established reimbursement framework to drive market 226
11.2.3 CANADA 230
11.2.3.1 Rising incidence of chronic and infectious lifestyle diseases
to propel market 230
11.3 EUROPE 235
11.3.1 MACROECONOMIC OUTLOOK FOR EUROPE 236
11.3.2 GERMANY 240
11.3.2.1 Increasing investments in clinical diagnostics research to drive market 240
11.3.3 UK 244
11.3.3.1 Rising adoption of genome-based testing to promote growth 244
11.3.4 FRANCE 248
11.3.4.1 High healthcare expenditure and rising investments in genomic
medicine to boost market 248
11.3.5 ITALY 251
11.3.5.1 Growing geriatric population and subsequent rise in chronic
conditions to support market growth 251
11.3.6 SPAIN 255
11.3.6.1 Rising adoption of immunoassay reagents to drive market 255
11.3.7 REST OF EUROPE 258
11.4 ASIA PACIFIC 263
11.4.1 MACROECONOMIC OUTLOOK FOR ASIA PACIFIC 263
11.4.2 JAPAN 268
11.4.2.1 Rise in social health insurance programs to encourage growth 268
11.4.3 CHINA 272
11.4.3.1 Growing focus on preventive care to boost demand 272
11.4.4 INDIA 276
11.4.4.1 Rising prevalence of chronic diseases to fuel market 276
11.4.5 REST OF ASIA PACIFIC 280
11.5 LATIN AMERICA 284
11.5.1 MACROECONOMIC OUTLOOK FOR LATIN AMERICA 284
11.5.2 BRAZIL 288
11.5.2.1 Rising prevalence of diabetes to stimulate growth 288
11.5.3 MEXICO 291
11.5.3.1 Growing establishment of clinical laboratories to boost market 291
11.5.4 REST OF LATIN AMERICA 295
11.6 MIDDLE EAST & AFRICA 298
11.6.1 GROWING FOCUS ON IMMUNOLOGY AND PRENATAL & CANCER TESTING
TO DRIVE MARKET 298
11.6.2 MACROECONOMIC OUTLOOK FOR MIDDLE EAST & AFRICA 299
11.7 GCC COUNTRIES 303
11.7.1 MACROECONOMIC OUTLOOK FOR GCC COUNTRIES 303
11.7.2 KINGDOM OF SAUDI ARABIA 307
11.7.2.1 Rising healthcare expenditure to promote growth 307
11.7.3 UNITED ARAB EMIRATES 310
11.7.3.1 Increasing development of advanced healthcare infrastructure
to expedite growth 310
11.7.4 OTHER GCC COUNTRIES 314
12 COMPETITIVE LANDSCAPE 318
12.1 OVERVIEW 318
12.2 KEY PLAYER STRATEGY/RIGHT TO WIN, 2023 318
12.2.1 OVERVIEW OF STRATEGIES ADOPTED BY KEY PLAYERS IN IVD REAGENTS
MARKET, 2021–2023 318
12.3 REVENUE ANALYSIS, 2021−2023 319
12.4 MARKET SHARE ANALYSIS, 2023 320
12.5 COMPANY VALUATION AND FINANCIAL METRICS 322
12.6 BRAND/PRODUCT COMPARISON 324
12.7 COMPANY EVALUATION MATRIX: KEY PLAYERS, 2023 325
12.7.1 STARS 325
12.7.2 EMERGING LEADERS 325
12.7.3 PERVASIVE PLAYERS 325
12.7.4 PARTICIPANTS 325
12.7.5 COMPANY FOOTPRINT: KEY PLAYERS, 2023 327
12.7.5.1 Company footprint 327
12.7.5.2 Technology footprint 328
12.7.5.3 Application footprint 329
12.7.5.4 Test type footprint 330
12.7.5.5 Region footprint 331
12.8 COMPANY EVALUATION MATRIX: STARTUPS/SMES, 2023 332
12.8.1 PROGRESSIVE COMPANIES 332
12.8.2 RESPONSIVE COMPANIES 332
12.8.3 DYNAMIC COMPANIES 332
12.8.4 STARTING BLOCKS 332
12.8.5 COMPETITIVE BENCHMARKING: STARTUPS/SMES, 2023 334
12.8.5.1 Detailed list of key startups/SMEs 334
12.8.5.2 Competitive benchmarking of key startups/SMEs 334
12.9 COMPETITIVE SCENARIO 335
12.9.1 PRODUCT LAUNCHES AND APPROVALS 335
12.9.2 DEALS 336
12.9.3 EXPANSIONS 337
12.9.4 OTHER DEVELOPMENTS 337
13 COMPANY PROFILES 338
13.1 KEY PLAYERS 338
13.1.1 DANAHER CORPORATION 338
13.1.1.1 Business overview 338
13.1.1.2 Products offered 339
13.1.1.3 Recent developments 340
13.1.1.3.1 Deals 340
13.1.1.3.2 Expansions 340
13.1.1.3.3 Other developments 340
13.1.1.4 MnM view 341
13.1.1.4.1 Right to win 341
13.1.1.4.2 Strategic choices 341
13.1.1.4.3 Weaknesses and competitive threats 341
13.1.2 F. HOFFMANN-LA ROCHE LTD. 342
13.1.2.1 Business overview 342
13.1.2.2 Products offered 343
13.1.2.3 Recent developments 344
13.1.2.3.1 Deals 344
13.1.2.4 MnM view 345
13.1.2.4.1 Right to win 345
13.1.2.4.2 Strategic choices 345
13.1.2.4.3 Weaknesses and competitive threats 345
13.1.3 ABBOTT 346
13.1.3.1 Business overview 346
13.1.3.2 Products offered 347
13.1.3.3 Recent developments 348
13.1.3.3.1 Deals 348
13.1.3.4 MnM view 349
13.1.3.4.1 Right to win 349
13.1.3.4.2 Strategic choices 349
13.1.3.4.3 Weaknesses and competitive threats 349
13.1.4 SIEMENS HEALTHINEERS AG 350
13.1.4.1 Business overview 350
13.1.4.2 Products offered 351
13.1.4.3 Recent developments 352
13.1.4.3.1 Deals 352
13.1.4.4 MnM view 353
13.1.4.4.1 Right to win 353
13.1.4.4.2 Strategic choices 353
13.1.4.4.3 Weaknesses and competitive threats 353
13.1.5 THERMO FISHER SCIENTIFIC INC. 354
13.1.5.1 Business overview 354
13.1.5.2 Products offered 355
13.1.5.3 Recent developments 356
13.1.5.3.1 Deals 356
13.1.5.3.2 Expansions 357
13.1.5.4 MnM view 358
13.1.5.4.1 Right to win 358
13.1.5.4.2 Strategic choices 358
13.1.5.4.3 Weaknesses and competitive threats 358
13.1.6 ILLUMINA, INC. 359
13.1.6.1 Business overview 359
13.1.6.2 Products offered 360
13.1.6.3 Recent developments 361
13.1.6.3.1 Deals 361
13.1.7 BIOMÉRIEUX 362
13.1.7.1 Business overview 362
13.1.7.2 Products offered 363
13.1.7.3 Recent developments 364
13.1.7.3.1 Deals 364
13.1.8 BD 365
13.1.8.1 Business overview 365
13.1.8.2 Products offered 367
13.1.8.3 Recent developments 367
13.1.8.3.1 Deals 367
13.1.9 HOLOGIC, INC. 368
13.1.9.1 Business overview 368
13.1.9.2 Products offered 370
13.1.9.3 Recent developments 371
13.1.9.3.1 Product launches and approvals 371
13.1.9.3.2 Deals 371
13.1.10 BIO-RAD LABORATORIES, INC. 372
13.1.10.1 Business overview 372
13.1.10.2 Products offered 374
13.1.10.3 Recent developments 374
13.1.10.3.1 Deals 374
13.1.11 SYSMEX CORPORATION 375
13.1.11.1 Business overview 375
13.1.11.2 Products offered 376
13.1.11.3 Recent developments 377
13.1.11.3.1 Product launches and approvals 377
13.1.11.3.2 Deals 377
13.1.11.3.3 Expansions 377
13.1.12 QIAGEN N.V. 378
13.1.12.1 Business overview 378
13.1.12.2 Products offered 379
13.1.12.3 Recent developments 380
13.1.12.3.1 Deals 380
13.1.13 AGILENT TECHNOLOGIES, INC. 381
13.1.13.1 Business overview 381
13.1.13.2 Products offered 383
13.1.13.3 Recent developments 384
13.1.13.3.1 Deals 384
13.1.13.3.2 Expansions 384
13.1.14 REVVITY 385
13.1.14.1 Business overview 385
13.1.14.2 Products offered 386
13.1.14.3 Recent developments 387
13.1.14.3.1 Deals 387
13.1.14.3.2 Other developments 387
13.1.15 DIASORIN S.P.A. 388
13.1.15.1 Business overview 388
13.1.15.2 Products offered 389
13.1.15.3 Recent developments 391
13.1.15.3.1 Deals 391
13.1.16 GRIFOLS, S.A. 392
13.1.16.1 Business overview 392
13.1.16.2 Products offered 393
13.1.16.3 Recent developments 394
13.1.16.3.1 Deals 394
13.1.17 WERFEN, S.A. 395
13.1.17.1 Business overview 395
13.1.17.2 Products offered 396
13.1.17.3 Recent developments 397
13.1.17.3.1 Deals 397
13.1.18 QUIDELORTHO CORPORATION 398
13.1.18.1 Business overview 398
13.1.18.2 Products offered 400
13.1.18.3 Recent developments 401
13.1.18.3.1 Deals 401
13.1.18.3.2 Expansions 401
13.1.19 CHEMBIO DIAGNOSTICS, INC. 402
13.1.19.1 Business overview 402
13.1.19.2 Products offered 403
13.1.20 SURMODICS, INC. 404
13.1.20.1 Business overview 404
13.1.20.2 Products offered 406
13.1.21 MERCK KGAA 407
13.1.21.1 Business overview 407
13.1.21.2 Products offered 408
13.2 OTHER PLAYERS 410
13.2.1 MEDICAL & BIOLOGICAL LABORATORIES CO., LTD. 410
13.2.2 CANVAX 411
13.2.3 PRESTIGE DIAGNOSTICS 412
13.2.4 ADALTIS S.R.L. 413
13.2.5 RANDOX LABORATORIES LTD. 414
14 APPENDIX 415
14.1 DISCUSSION GUIDE 415
14.2 KNOWLEDGESTORE: MARKETSANDMARKETS’ SUBSCRIPTION PORTAL 420
14.3 CUSTOMIZATION OPTIONS 422
14.4 RELATED REPORTS 422
14.5 AUTHOR DETAILS 423
❖ 世界のIVD試薬市場に関するよくある質問(FAQ) ❖
・IVD試薬の世界市場規模は?
→MarketsandMarkets社は2024年のIVD試薬の世界市場規模を604.5億ドルと推定しています。
・IVD試薬の世界市場予測は?
→MarketsandMarkets社は2029年のIVD試薬の世界市場規模を874.1億ドルと予測しています。
・IVD試薬市場の成長率は?
→MarketsandMarkets社はIVD試薬の世界市場が2024年~2029年に年平均7.7%成長すると予測しています。
・世界のIVD試薬市場における主要企業は?
→MarketsandMarkets社は「Danaher Corporation (US), F. Hoffmann-La Roche Ltd (Switzerland), Abbott (US), Siemens Healthineers AG (Germany), Thermo Fisher Scientific Inc. (US), Illumina, Inc. (US), BioMerieux (France), BD (US), Hologic, Inc. (US), Bio-Rad Laboratories, Inc. (US), Sysmex Corporation (Japan), QIAGEN N.V. (Netherlands), Agilent Technologies, Inc. (US), Revvity (US), DiaSorin S.p.A (Italy), Grifols, S.A. (Spain), Werfen, S.A. (Spain), QuidelOrtho Corporation (US), Chembio Diagnostics, Inc. (US), Surmodics, Inc. (US), Merck KGaA (Germany), MEDICAL & BIOLOGICAL LABORATORIES CO., LTD. (Tokyo), Canvax (Spain), Prestige Diagnostics (US), Adaltis S.r.l. (Italy), and Randox Laboratories Ltd. (UK)など ...」をグローバルIVD試薬市場の主要企業として認識しています。
※上記FAQの市場規模、市場予測、成長率、主要企業に関する情報は本レポートの概要を作成した時点での情報であり、納品レポートの情報と少し異なる場合があります。











