1 はじめに
1.1 調査目的 25
1.2 市場の定義 25
1.3 市場範囲 26
1.3.1 対象市場 26
1.3.2 対象範囲と対象外 27
1.3.3 考慮した年数 27
1.3.4 通貨
1.4 利害関係者 28
1.5 変更点のまとめ 28
2 調査方法 29
2.1 調査データ 29
2.2 調査デザイン 30
2.2.1 二次調査 30
2.2.2 一次調査 32
2.2.2.1 一次情報源 33
2.2.2.2 主要な業界インサイト 34
2.2.2.3 一次調査の内訳 34
2.3 市場規模の推定 35
2.3.1 ボトムアップアプローチ 36
2.3.1.1 アプローチ1:企業収益推定アプローチ 37
2.3.1.2 アプローチ2:顧客ベースの市場推定 38
2.3.1.3 アプローチ3:トップダウンアプローチ 39
2.3.1.4 アプローチ4:プライマリーインタビュー 40
2.3.1.5 CAGR予測 41
2.4 データの三角測量と市場の内訳 42
2.5 市場シェア評価 43
2.6 調査の前提 43
2.7 前提条件/市場予測方法 43
2.8 限界とリスク評価 44
3 エグゼクティブサマリー
4 プレミアムインサイト 49
4.1 抗菌薬感受性試験市場の概要 49
4.2 抗菌薬感受性試験市場、
地域別、2024年対2029年(百万米ドル) 50
4.3 抗菌薬感受性試験市場、
地域・エンドユーザー別、2023年(百万米ドル) 51
4.4 抗菌薬感受性試験市場における地理的成長機会
市場成長機会
5 市場の概要
5.1 はじめに
5.2 市場ダイナミクス
5.2.1 推進要因 54
5.2.1.1 抗菌薬耐性の増加 54
5.2.1.2 診断技術の進歩 56
5.2.1.3 臨床研究アプリケーションの拡大 57
5.2.1.4 政府のイニシアチブの高まり 58
5.2.2 阻害要因 58
5.2.2.1 自動化機器の高コスト 58
5.2.3 機会 59
5.2.3.1 迅速検査ソリューションの出現 59
5.2.3.2 デジタルヘルス技術との統合 60
5.2.4 課題 61
5.2.4.1 複雑な規制環境 61
5.3 業界動向 62
5.3.1 等温マイクロカロリメトリー 62
5.3.2 蛍光活性化セルソーティング 63
5.3.3 スマートフォンベースの光学分光法 63
5.3.4 マイクロフルイディクスとマイクロドロップレット 63
5.4 エコシステム分析 64
5.5 サプライチェーン分析 65
5.5.1 著名企業 65
5.5.2 中小企業 65
5.5.3 エンドユーザー 65
5.6 バリューチェーン分析 66
5.7 ポーターの5つの力分析 68
5.7.1 新規参入企業の脅威 69
5.7.2 供給者の交渉力 69
5.7.3 買い手の交渉力 69
5.7.4 代替品の脅威 69
5.7.5 競合の激しさ 69
5.8 主要ステークホルダーと購買基準 70
5.8.1 購入プロセスにおける主要ステークホルダー 70
5.8.2 購入基準
5.9 特許分析 72
5.10 貿易分析 75
5.10.1 輸入データ 75
5.10.2 輸出データ 76
5.11 主要な会議とイベント(2024-2025年) 77
5.12 顧客のビジネスに影響を与えるトレンド/混乱 78
5.13 技術分析 78
5.13.1 主要技術 79
5.13.1.1 PCR 79
5.13.1.2 マイクロ流体工学とラボオンチップ 79
5.13.1.3 合成抗菌ペプチド 79
5.13.2 補完技術 79
5.13.2.1 マルディトフ 79
5.13.3 隣接技術 80
5.13.3.1 ナノテクノロジー 80
5.13.3.2 ナノモーション技術 80
5.13.3.3 バイオチップ技術 80
5.14 規制の状況 81
5.14.1 北米 81
5.14.1.1 米国 81
5.14.1.2 カナダ 82
5.14.2 欧州 82
5.14.3 アジア太平洋地域 83
5.14.3.1 日本 83
5.14.3.2 中国 84
5.14.3.3 インド 84
5.14.4 規制機関、政府機関、
その他の組織 85
5.15 投資と資金調達のシナリオ 87
5.16 価格分析 88
5.16.1 平均販売価格(地域別) 88
5.16.2 平均販売価格、主要プレーヤー別 92
5.17償還シナリオ 92
5.18 アンメットニーズと主要ペインポイント 94
5.19 抗菌薬感受性試験市場におけるAI/GEN AIの影響 94
6 抗菌薬感受性試験市場:製品別 96
6.1 導入 97
6.2 消耗品 97
6.2.1 培地および増殖培地 98
6.2.1.1 ディスク拡散法とブロス希釈法の高い利用率が市場を牽引 98
市場を牽引 98
6.2.2 感受性試験ディスク 99
6.2.2.1 低コスト、使いやすさが普及を後押し 99
6.2.3 マイクストリップ 99
6.2.3.1 正確性、簡便性、詳細な結果が利用を促進 99
6.2.4 感受性試験用プレート 100
6.2.4.1 専用プレートの入手可能性が市場を牽引 100
6.3 検査機器 101
6.3.1 自動化された検査機器 102
6.3.1.1 検査室の自動化傾向の継続がセグメント成長を促進 102
6.3.2 分子診断システム 103
6.3.2.1 分子診断の技術的進歩が市場を促進 103
が市場を牽引 103
6.4 研究用消耗品 104
6.4.1 病院で実施される感受性検査の増加が需要を牽引 104
7 抗菌薬感受性試験市場:タイプ別 105
7.1 導入 106
7.2 抗菌薬感受性試験 106
7.2.1 多剤耐性の出現が抗菌薬感受性検査製品の採用を増加させる 106
7.3 抗真菌剤感受性試験 107
7.3.1 感染症の流行増加が検査需要を促進 107
7.4 抗寄生虫感受性試験 109
7.4.1 市場成長を支える意識の高まり 109
7.5 抗ウイルス剤感受性試験 110
7.5.1 患者の転帰を向上させる抗ウイルス剤感受性検査の進歩 110
8 抗菌薬感受性試験市場:方法別 111
8.1 導入 112
8.2 定性試験法 112
8.2.1 自動化されたast 113
8.2.1.1 自動化機器の使用を促進する進歩と耐性パターン 113
8.2.2 ディスク拡散法 115
8.2.2.1 簡便性、正確性、柔軟性が採用を後押し 115
8.2.3 寒天希釈法 116
8.2.3.1 採用に影響する時間と労力のかかる性質 116
8.2.4 遺伝子型分析法 117
8.2.4.1 ゴールドスタンダードの地位が採用拡大に寄与 117
8.3 定量的方法 118
8.3.1 エテスト法 119
8.3.1.1 定量法市場ではEtest法が大きなシェアを占める 119
8.3.2 ブロスマクロダイリューション 120
8.3.2.1 高精度、同時検査機能が市場を牽引 120
9 抗菌薬感受性試験市場:用途別 121
9.1 はじめに 122
9.2 臨床診断 123
9.2.1 技術的進歩が市場を牽引 123
9.3 創薬・医薬品開発 124
9.3.1 需要拡大を支える研究開発とイノベーションの重視 124
9.4 疫学 125
9.4.1 より良い理解と意思決定の必要性が採用を促進 125
9.5 その他のアプリケーション 126
10 抗菌薬感受性試験市場:エンドユーザー別 127
10.1 導入 128
10.2 病院・診断センター 128
10.2.1 病院・診断センターが最大市場シェアを占める 128
10.3 製薬・バイオテクノロジー企業 129
10.3.1 製品開発の重視が市場を牽引 129
10.4 学術・研究機関 130
10.4.1 研究開発活動の活発化と研究支援が市場を牽引 130
10.5 臨床研究機関 132
10.5.1 世界的な臨床試験の拡大が市場成長を促進 132
11 抗菌薬感受性試験市場(地域別) 133
11.1 はじめに 134
11.2 北米 135
11.2.1 北米:マクロ経済見通し 135
11.2.2 米国 140
11.2.2.1 北米市場で最大のシェアを占める米国 140
11.2.3 カナダ 142
11.2.3.1 政府の好意的な取り組みが市場成長を促進 142
11.3 欧州 144
11.3.1 欧州: マクロ経済見通し 144
11.3.2 ドイツ 149
11.3.2.1 AMRに対する政府の取り組みが市場を牽引 149
11.3.3 フランス 150
11.3.3.1 AMRサーベイランスの強化が市場を牽引 150
11.3.4 英国 152
11.3.4.1 AMRに関する新たな行動計画が普及を促進 152
11.3.5 イタリア 153
11.3.5.1 AMR患者の増加と抗生物質の大量使用が検査需要を促進 153
11.3.6 スペイン 155
11.3.6.1 グラム陰性桿菌におけるAMRの増加が市場を牽引 155
11.3.7 その他の欧州 157
11.4 アジア太平洋地域 158
11.4.1 アジア太平洋地域:マクロ経済見通し 159
11.4.2 日本 164
11.4.2.1 感染症管理のための産官学連携が市場成長を支える 164
11.4.3 中国 166
11.4.3.1 AMR管理重視の高まりが市場を牽引 166
11.4.4 インド 168
11.4.4.1 臨床診断における政府と業界の取り組みが市場成長を促進 168
11.4.5 オーストラリア 170
11.4.5.1 抗菌薬治療ガイドラインの確立と実施が市場成長を促進 170
11.4.6 韓国 172
11.4.6.1 迅速なAMR検出の必要性が自動ASTシステムの需要を促進 172
11.4.7 その他のアジア太平洋地域 173
11.5 ラテンアメリカ 175
11.5.1 ラテンアメリカ:マクロ経済見通し 175
11.5.2 ブラジル 179
11.5.2.1 AMR検出の実施重視の高まりが市場を牽引 179
11.5.3 メキシコ 181
11.5.3.1 ASTシステムの拡大を支える経済成長 181
11.5.4 その他のラテンアメリカ 182
11.6 中東・アフリカ 183
11.6.1 中東・アフリカ:マクロ経済見通し 184
11.6.2 北アフリカ諸国 188
11.6.2.1 成長を支える医療インフラ開発イニシアティブ 188
11.6.3 その他の中東・アフリカ 189
12 競争環境 191
12.1 概要 191
12.2 主要プレーヤーの戦略/勝利への権利 191
12.2.1 抗菌薬感受性試験市場で各社が採用した戦略の概要 192
12.3 収益分析 193
12.4 市場シェア分析、2023年 194
12.5 企業評価マトリックス:主要企業(2023年) 196
12.5.1 スター企業 196
12.5.2 新興リーダー 196
12.5.3 浸透型プレーヤー 196
12.5.4 参加企業 196
12.5.5 企業フットプリント:主要プレーヤー(2023年) 198
12.5.5.1 企業フットプリント 198
12.5.5.2 地域別フットプリント 199
12.5.5.3 製品フットプリント 200
12.5.5.4 タイプ別フットプリント 201
12.5.5.5 メソッドフットプリント 202
12.5.5.6 アプリケーションフットプリント 203
12.5.5.7 エンドユーザーフットプリント 204
12.6 企業評価マトリクス:新興企業/SM(2023年) 205
12.6.1 進歩的企業 205
12.6.2 対応力のある企業 205
12.6.3 ダイナミックな企業 205
12.6.4 スタートアップ企業 205
12.6.5 競争ベンチマーク:新興企業/SM(2023年) 207
12.7 評価と財務指標 209
12.7.1 財務指標 209
12.7.2 企業評価 209
12.8 ブランド/製品の比較 210
12.9 競争シナリオ 211
12.9.1 製品の上市と承認 211
12.9.2 取引 212
13 会社プロファイル 213
BioMérieux (France)
Becton
Dickinson and company (US)
Thermo Fisher Scientific Inc. (US)
Danaher Corporation (US)
Bio-Rad laboratories (US)
14 付録 274
14.1 ディスカッションガイド 274
14.2 Knowledgestore: Marketsandmarketsの購読ポータル 279
14.3 カスタマイズオプション 281
14.4 関連レポート 281
14.5 著者の詳細 282
Key advantages of the AST market include the fact that it is very crucial in improving patient care through proper identification and timely selection of effective antimicrobial therapy. AST therefore has an added advantage in combating the rise of the antimicrobial resistance problem in providing correct susceptibility profiles that assist appropriate antibiotic usage.
“Consumables to register largest market share in 2022-2029.”
The largest share in antimicrobial susceptibility testing (AST) is held by the consumables segment. A significant and ever-increasing amount of critical material is consumed. It comprises culture media, reagents, antibiotic discs, and other materials and can be considered basically necessary for AST procedures. The market share of the consumable is thus added to owing to the growing incidence of infectious diseases and the emergence of antibiotic-resistant pathogens and increasing demand for AST services.
Second, the production of more sophisticated AST technologies will need more technical consumables. Obviously, molecular-based AST methods will require specific nucleic acid extraction kits, PCR reagents, and detection probes. Persistent demand for accurate and effective AST testing creates a sustainable demand for consumables, which further solidifies them as the core of the overall AST market.
“Hospitals & diagnostic centers segment held the largest share of antimicrobial susceptibility testingmarket in 2023, by End-user.”
Based on the end-user, the antimicrobial susceptibility testing market is segmented into hospitals & diagnostic laboratories, pharmaceutical and biotechnology companies, research & academic Institutes and clinical research organizations. In the year 2023, the global antimicrobial susceptibility testing market was dominated by hospitals and diagnostic centers. Growth within the number of hospitals and diagnostic centers in the AST market is also derived from increases in the timely and accurate diagnostics needs for managing infectious diseases and fighting against antimicrobial resistance.
"Asia Pacific to register highest growth rate in the market during the forecast period."
The Asia Pacific region, and China in particular, is witnessing a very rapid growth in the AST market due to various reasons. The increasing population in this region, allied with increasing incomes and access to better health care, has resulted in more stringent demands for diagnostics services. It is not overstressed in the sense that the ever-growing occurrence of infectious diseases and antimicrobial-resistant pathogens demands utmost importance in attaching real value to accurate AST as the primary basis for guiding proper treatment.
Advances in AST technology, particularly through the development of molecular rapid diagnostics and automated systems, is driving the market.. These speed up the process of AST, give results quicker in turn-around time, have increased efficiency with precise results, which put AST within the reach of healthcare providers. Additionally, the investments done by governments in upgrading the health care infrastructure along with such initiatives have paved a favorable path toward the growth of the market in the country, particularly in China.
A number of drivers were responsible for the upswing, including:
A breakdown of the primary participants referred to for this report is provided below:
• By Company Type: Tier 1–30%, Tier 2–42%, and Tier 3– 28%
• By Designation: C-level-- 10%, Director-level–14%, and Others–76%
• By Region: North America–40%, Europe-30%, Asia Pacific–20%, Latin America- 5%, Middle east and africa- 5%
Prominent players in this market are BioMérieux (France), Becton, Dickinson and company (US), Thermo Fisher Scientific Inc. (US), Danaher Corporation (US), Bio-Rad laboratories (US), Bruker (US), Roche diagnostics (Switzerland, Accelerate diagnostics (US), Himedia laboratories (India), Liofilchem s.r.l.(Italy), Alifax s.r.l.(Italy), Creative Diagnostics (US), among others.
Research Coverage
The market is segmented into product, type, method, application, end user, and region. In the report, factors affecting the antimicrobial susceptibility testing market- drivers, restraints, opportunities, and challenges-are described. Opportunities and challenges for stakeholders and the details of leading players' competitive landscape are also highlighted. The report further segments the micro-markets into growth trends, prospects, and contribution to the antimicrobial susceptibility testing market. It forecasts revenue growth from different market segments, focusing on five major geographical regions.
Key Benefits of Buying the Report:
The report is designed to aid new entrants by providing them with detailed data on the antimicrobial susceptibility testing market, thereby allowing them to understand investment opportunities. It provides comprehensive insight into key players as well as smaller ones, thus favoring strong risk assessment and informed investment decisions. Due to precise segmentation-such as by end-users and regions-the report offers focused insights in specific segments of the market. It further outlines the critical trends, challenges, growth drivers, and opportunities that complete the strategic decision-making process with well-rounded analysis.
The report provides the insights on the following pointers:
Analysis of the key drivers, restraints, opportunities, and challenges influencing the rise of the antimicrobial susceptibility testing market. The key drivers of antimicrobial susceptibility testing are an upsurge in infectious diseases and, correspondingly, higher numbers of antibiotic resistance around the world. Growing demand for timely and accurate diagnostic tools that guide effective treatment strategies fuels demand for AST. Technologically, the development of rapid and automated testing systems supports market growth by increasing efficiency and accuracy in tests.
Product Development/Innovation: Insights into emerging technologies, current R&D activities, and recent launches of products and services in the antimicrobial susceptibility testing market.
Market Development: The report further provides in detail the profitable markets by segmenting the antimicrobial susceptibility testing market into various regions
Market Diversification: Detailed insight into new product launches, unexplored markets, recent developments, and investments made in the antimicrobial susceptibility testing market.
Competitive Assessment: Detailed assessment of market share, service offerings leading strategies of key players such as BioMérieux (France), Becton, Dickinson and company (US), Thermo Fisher Scientific Inc. (US), Danaher Corporation (US), Bio-Rad laboratories (US), among others.
1 INTRODUCTION 25
1.1 STUDY OBJECTIVES 25
1.2 MARKET DEFINITION 25
1.3 MARKET SCOPE 26
1.3.1 MARKETS COVERED 26
1.3.2 INCLUSIONS AND EXCLUSIONS 27
1.3.3 YEARS CONSIDERED 27
1.3.4 CURRENCY CONSIDERED 27
1.4 STAKEHOLDERS 28
1.5 SUMMARY OF CHANGES 28
2 RESEARCH METHODOLOGY 29
2.1 RESEARCH DATA 29
2.2 RESEARCH DESIGN 30
2.2.1 SECONDARY RESEARCH 30
2.2.2 PRIMARY RESEARCH 32
2.2.2.1 Primary sources 33
2.2.2.2 Key industry insights 34
2.2.2.3 Breakdown of primaries 34
2.3 MARKET SIZE ESTIMATION 35
2.3.1 BOTTOM-UP APPROACH 36
2.3.1.1 Approach 1: Company revenue estimation approach 37
2.3.1.2 Approach 2: Customer-based market estimation 38
2.3.1.3 Approach 3: Top-down approach 39
2.3.1.4 Approach 4: Primary interviews 40
2.3.1.5 CAGR projections 41
2.4 DATA TRIANGULATION & MARKET BREAKDOWN 42
2.5 MARKET SHARE ASSESSMENT 43
2.6 STUDY ASSUMPTIONS 43
2.7 ASSUMPTIONS/MARKET FORECASTING METHODOLOGY 43
2.8 LIMITATIONS AND RISK ASSESSMENT 44
3 EXECUTIVE SUMMARY 45
4 PREMIUM INSIGHTS 49
4.1 ANTIMICROBIAL SUSCEPTIBILITY TESTING MARKET OVERVIEW 49
4.2 ANTIMICROBIAL SUSCEPTIBILITY TESTING MARKET,
BY REGION, 2024 VS. 2029 (USD MILLION) 50
4.3 ANTIMICROBIAL SUSCEPTIBILITY TESTING MARKET,
BY REGION AND END USER, 2023 (USD MILLION) 51
4.4 GEOGRAPHIC GROWTH OPPORTUNITIES IN ANTIMICROBIAL SUSCEPTIBILITY
TESTING MARKET 52
5 MARKET OVERVIEW 53
5.1 INTRODUCTION 53
5.2 MARKET DYNAMICS 54
5.2.1 DRIVERS 54
5.2.1.1 Rising antimicrobial resistance 54
5.2.1.2 Advancements in diagnostic technologies 56
5.2.1.3 Expansion of clinical research applications 57
5.2.1.4 Rise in government initiatives 58
5.2.2 RESTRAINTS 58
5.2.2.1 High cost of automated instruments 58
5.2.3 OPPORTUNITIES 59
5.2.3.1 Emergence of rapid testing solutions 59
5.2.3.2 Integration with digital health technologies 60
5.2.4 CHALLENGES 61
5.2.4.1 Intricate regulatory landscape 61
5.3 INDUSTRY TRENDS 62
5.3.1 ISOTHERMAL MICROCALORIMETRY 62
5.3.2 FLUORESCENCE-ACTIVATED CELL SORTING 63
5.3.3 SMARTPHONE-BASED OPTICAL SPECTROSCOPY 63
5.3.4 MICROFLUIDICS AND MICRODROPLETS 63
5.4 ECOSYSTEM ANALYSIS 64
5.5 SUPPLY CHAIN ANALYSIS 65
5.5.1 PROMINENT COMPANIES 65
5.5.2 SMALL AND MEDIUM-SIZED ENTERPRISES 65
5.5.3 END USERS 65
5.6 VALUE CHAIN ANALYSIS 66
5.7 PORTER’S FIVE FORCES ANALYSIS 68
5.7.1 THREAT OF NEW ENTRANTS 69
5.7.2 BARGAINING POWER OF SUPPLIERS 69
5.7.3 BARGAINING POWER OF BUYERS 69
5.7.4 THREAT OF SUBSTITUTES 69
5.7.5 INTENSITY OF COMPETITIVE RIVALRY 69
5.8 KEY STAKEHOLDERS AND BUYING CRITERIA 70
5.8.1 KEY STAKEHOLDERS IN BUYING PROCESS 70
5.8.2 BUYING CRITERIA 71
5.9 PATENT ANALYSIS 72
5.10 TRADE ANALYSIS 75
5.10.1 IMPORT DATA 75
5.10.2 EXPORT DATA 76
5.11 KEY CONFERENCES AND EVENTS, 2024–2025 77
5.12 TRENDS/DISRUPTIONS IMPACTING CUSTOMERS’ BUSINESSES 78
5.13 TECHNOLOGY ANALYSIS 78
5.13.1 KEY TECHNOLOGIES 79
5.13.1.1 PCR 79
5.13.1.2 Microfluidics and lab-on-a-chip 79
5.13.1.3 Synthetic antimicrobial peptides 79
5.13.2 COMPLIMENTARY TECHNOLOGIES 79
5.13.2.1 MALDI-TOF 79
5.13.3 ADJACENT TECHNOLOGIES 80
5.13.3.1 Nanotechnology 80
5.13.3.2 Nanomotion technology 80
5.13.3.3 Biochip technology 80
5.14 REGULATORY LANDSCAPE 81
5.14.1 NORTH AMERICA 81
5.14.1.1 US 81
5.14.1.2 Canada 82
5.14.2 EUROPE 82
5.14.3 ASIA PACIFIC 83
5.14.3.1 Japan 83
5.14.3.2 China 84
5.14.3.3 India 84
5.14.4 REGULATORY BODIES, GOVERNMENT AGENCIES,
AND OTHER ORGANIZATIONS 85
5.15 INVESTMENT AND FUNDING SCENARIO 87
5.16 PRICING ANALYSIS 88
5.16.1 AVERAGE SELLING PRICE, BY REGION 88
5.16.2 AVERAGE SELLING PRICE, BY KEY PLAYER 92
5.17 REIMBURSEMENT SCENARIO 92
5.18 UNMET NEEDS AND KEY PAIN POINTS 94
5.19 IMPACT OF AI/GEN AI ON ANTIMICROBIAL SUSCEPTIBILITY TESTING MARKET 94
6 ANTIMICROBIAL SUSCEPTIBILITY TESTING MARKET, BY PRODUCT 96
6.1 INTRODUCTION 97
6.2 CONSUMABLES 97
6.2.1 CULTURE & GROWTH MEDIA 98
6.2.1.1 High utilization of disk diffusion and broth dilution methods
to drive market 98
6.2.2 SUSCEPTIBILITY TESTING DISKS 99
6.2.2.1 Low costs, ease of use to support adoption 99
6.2.3 MIC STRIPS 99
6.2.3.1 Accuracy, simplicity, and thorough results to drive usage 99
6.2.4 SUSCEPTIBILITY TESTING PLATES 100
6.2.4.1 Availability of specialized plates to drive market 100
6.3 LAB INSTRUMENTS 101
6.3.1 AUTOMATED LABORATORY INSTRUMENTS 102
6.3.1.1 Ongoing trend of laboratory automation to fuel segment growth 102
6.3.2 MOLECULAR DIAGNOSTICS SYSTEMS 103
6.3.2.1 Technological advancements in molecular diagnostics
to propel market 103
6.4 LAB DISPOSABLES 104
6.4.1 INCREASING NUMBER OF SUSCEPTIBILITY TESTS PERFORMED IN HOSPITALS TO DRIVE DEMAND 104
7 ANTIMICROBIAL SUSCEPTIBILITY TESTING MARKET, BY TYPE 105
7.1 INTRODUCTION 106
7.2 ANTIBACTERIAL SUSCEPTIBILITY TESTING 106
7.2.1 EMERGENCE OF MULTIDRUG-RESISTANCE TO INCREASE ADOPTION OF ANTIBACTERIAL SUSCEPTIBILITY TESTING PRODUCTS 106
7.3 ANTIFUNGAL SUSCEPTIBILITY TESTING 107
7.3.1 INCREASING PREVALENCE OF INFECTIOUS DISEASES TO PROPEL DEMAND FOR TESTING 107
7.4 ANTIPARASITIC SUSCEPTIBILITY TESTING 109
7.4.1 RISING AWARENESS TO SUPPORT MARKET GROWTH 109
7.5 ANTIVIRAL SUSCEPTIBILITY TESTING 110
7.5.1 ADVANCEMENTS IN ANTIVIRAL SUSCEPTIBILITY TESTING TO ENHANCE PATIENT OUTCOMES 110
8 ANTIMICROBIAL SUSCEPTIBILITY TESTING MARKET, BY METHOD 111
8.1 INTRODUCTION 112
8.2 QUALITATIVE METHODS 112
8.2.1 AUTOMATED AST 113
8.2.1.1 Advancements and resistance patterns to drive use of automated instruments 113
8.2.2 DISK DIFFUSION 115
8.2.2.1 Simplicity, accuracy, and flexibility to support adoption 115
8.2.3 AGAR DILUTION 116
8.2.3.1 Time-consuming and labor-intensive nature to affect adoption 116
8.2.4 GENOTYPIC METHOD 117
8.2.4.1 Gold standard status to support greater adoption 117
8.3 QUANTITATIVE METHODS 118
8.3.1 ETEST METHOD 119
8.3.1.1 Etest to hold larger share of quantitative methods market 119
8.3.2 BROTH MACRODILUTION 120
8.3.2.1 Precision, simultaneous testing capability to drive market 120
9 ANTIMICROBIAL SUSCEPTIBILITY TESTING MARKET, BY APPLICATION 121
9.1 INTRODUCTION 122
9.2 CLINICAL DIAGNOSTICS 123
9.2.1 TECHNOLOGICAL ADVANCEMENTS TO DRIVE MARKET 123
9.3 DRUG DISCOVERY & DEVELOPMENT 124
9.3.1 EMPHASIS ON R&D AND INNOVATION TO SUPPORT DEMAND GROWTH 124
9.4 EPIDEMIOLOGY 125
9.4.1 NEED FOR BETTER UNDERSTANDING AND DECISION MAKING TO PROPEL ADOPTION 125
9.5 OTHER APPLICATIONS 126
10 ANTIMICROBIAL SUSCEPTIBILITY TESTING MARKET, BY END USER 127
10.1 INTRODUCTION 128
10.2 HOSPITALS & DIAGNOSTIC CENTERS 128
10.2.1 HOSPITALS & DIAGNOSTIC CENTERS TO HOLD LARGEST MARKET SHARE 128
10.3 PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES 129
10.3.1 EMPHASIS ON PRODUCT DEVELOPMENT TO DRIVE MARKET 129
10.4 ACADEMIC & RESEARCH INSTITUTES 130
10.4.1 RISING R&D ACTIVITY AND SUPPORT FOR RESEARCH TO DRIVE MARKET 130
10.5 CLINICAL RESEARCH ORGANIZATIONS 132
10.5.1 EXPANSION OF CLINICAL TRIALS WORLDWIDE TO PROPEL MARKET GROWTH 132
11 ANTIMICROBIAL SUSCEPTIBILITY TESTING MARKET, BY REGION 133
11.1 INTRODUCTION 134
11.2 NORTH AMERICA 135
11.2.1 NORTH AMERICA: MACROECONOMIC OUTLOOK 135
11.2.2 US 140
11.2.2.1 US to hold largest share of North American market 140
11.2.3 CANADA 142
11.2.3.1 Favorable government initiatives to drive market growth 142
11.3 EUROPE 144
11.3.1 EUROPE: MACROECONOMIC OUTLOOK 144
11.3.2 GERMANY 149
11.3.2.1 Government initiatives for AMR to drive market 149
11.3.3 FRANCE 150
11.3.3.1 Focus on enhancing AMR surveillance to propel market 150
11.3.4 UK 152
11.3.4.1 New action plans for AMR to propel adoption 152
11.3.5 ITALY 153
11.3.5.1 Rising instances of AMR and high use of antibiotics to propel demand for testing 153
11.3.6 SPAIN 155
11.3.6.1 Rising AMR in gram-negative bacilli to drive market 155
11.3.7 REST OF EUROPE 157
11.4 ASIA PACIFIC 158
11.4.1 ASIA PACIFIC: MACROECONOMIC OUTLOOK 159
11.4.2 JAPAN 164
11.4.2.1 Industry-government collaborations for infectious disease management to support market growth 164
11.4.3 CHINA 166
11.4.3.1 Rising emphasis on AMR management to drive market 166
11.4.4 INDIA 168
11.4.4.1 Expanding government and industry efforts in clinical diagnostics to propel market growth 168
11.4.5 AUSTRALIA 170
11.4.5.1 Establishment and implementation of antimicrobial treatment guidelines to favor market growth 170
11.4.6 SOUTH KOREA 172
11.4.6.1 Need for rapid AMR detection to drive demand for automated AST systems 172
11.4.7 REST OF ASIA PACIFIC 173
11.5 LATIN AMERICA 175
11.5.1 LATIN AMERICA: MACROECONOMIC OUTLOOK 175
11.5.2 BRAZIL 179
11.5.2.1 Rising emphasis on implementing AMR detection to drive market 179
11.5.3 MEXICO 181
11.5.3.1 Economic growth to support expansion of AST systems 181
11.5.4 REST OF LATIN AMERICA 182
11.6 MIDDLE EAST & AFRICA 183
11.6.1 MIDDLE EAST & AFRICA: MACROECONOMIC OUTLOOK 184
11.6.2 GCC COUNTRIES 188
11.6.2.1 Healthcare infrastructural development initiatives to support growth 188
11.6.3 REST OF MIDDLE EAST & AFRICA 189
12 COMPETITIVE LANDSCAPE 191
12.1 OVERVIEW 191
12.2 KEY PLAYER STRATEGIES/RIGHT TO WIN 191
12.2.1 OVERVIEW OF STRATEGIES ADOPTED BY PLAYERS IN ANTIMICROBIAL SUSCEPTIBILITY TESTING MARKET 192
12.3 REVENUE ANALYSIS 193
12.4 MARKET SHARE ANALYSIS, 2023 194
12.5 COMPANY EVALUATION MATRIX: KEY PLAYERS, 2023 196
12.5.1 STARS 196
12.5.2 EMERGING LEADERS 196
12.5.3 PERVASIVE PLAYERS 196
12.5.4 PARTICIPANTS 196
12.5.5 COMPANY FOOTPRINT: KEY PLAYERS, 2023 198
12.5.5.1 Company footprint 198
12.5.5.2 Region footprint 199
12.5.5.3 Product footprint 200
12.5.5.4 Type footprint 201
12.5.5.5 Method footprint 202
12.5.5.6 Application footprint 203
12.5.5.7 End-user footprint 204
12.6 COMPANY EVALUATION MATRIX: STARTUPS/SMES, 2023 205
12.6.1 PROGRESSIVE COMPANIES 205
12.6.2 RESPONSIVE COMPANIES 205
12.6.3 DYNAMIC COMPANIES 205
12.6.4 STARTING BLOCKS 205
12.6.5 COMPETITIVE BENCHMARKING: STARTUPS/SMES, 2023 207
12.7 VALUATION & FINANCIAL METRICS 209
12.7.1 FINANCIAL METRICS 209
12.7.2 COMPANY VALUATION 209
12.8 BRAND/PRODUCT COMPARISON 210
12.9 COMPETITIVE SCENARIO 211
12.9.1 PRODUCT LAUNCHES & APPROVALS 211
12.9.2 DEALS 212
13 COMPANY PROFILES 213
13.1 KEY PLAYERS 213
13.1.1 BIOMÉRIEUX SA 213
13.1.1.1 Business overview 213
13.1.1.2 Products offered 214
13.1.1.3 Recent developments 216
13.1.1.3.1 Product launches & approvals 216
13.1.1.3.2 Deals 217
13.1.1.4 MnM view 217
13.1.1.4.1 Right to win 217
13.1.1.4.2 Strategic choices 217
13.1.1.4.3 Weaknesses & competitive threats 217
13.1.2 BECTON, DICKINSON AND COMPANY 218
13.1.2.1 Business overview 218
13.1.2.2 Products offered 220
13.1.2.3 Recent developments 221
13.1.2.3.1 Deals 221
13.1.2.3.2 Other developments 221
13.1.2.4 MnM view 221
13.1.2.4.1 Right to win 221
13.1.2.4.2 Strategic choices 222
13.1.2.4.3 Weaknesses and competitive threats 222
13.1.3 THERMO FISHER SCIENTIFIC INC. 223
13.1.3.1 Business overview 223
13.1.3.2 Products offered 224
13.1.3.3 Recent developments 226
13.1.3.3.1 Product launches & approvals 226
13.1.3.3.2 Deals 226
13.1.3.4 MnM view 227
13.1.3.4.1 Right to win 227
13.1.3.4.2 Strategic choices 227
13.1.3.4.3 Weaknesses and competitive threats 227
13.1.4 DANAHER CORPORATION 228
13.1.4.1 Business overview 228
13.1.4.2 Products offered 230
13.1.4.3 Recent developments 231
13.1.4.3.1 Product launches & approvals 231
13.1.4.3.2 Deals 232
13.1.4.4 MnM view 233
13.1.4.4.1 Right to win 233
13.1.4.4.2 Strategic choices 233
13.1.4.4.3 Weaknesses and competitive threats 233
13.1.5 BIO-RAD LABORATORIES, INC. 234
13.1.5.1 Business overview 234
13.1.5.2 Products offered 235
13.1.5.2.1 Deals 236
13.1.5.3 MnM view 236
13.1.5.3.1 Right to win 236
13.1.5.3.2 Strategic choices 236
13.1.5.3.3 Weaknesses and competitive threats 236
13.1.6 BRUKER 237
13.1.6.1 Business overview 237
13.1.6.2 Products offered 239
13.1.6.3 Recent developments 241
13.1.6.3.1 Product launches & approvals 241
13.1.6.3.2 Deals 241
13.1.7 ROCHE DIAGNOSTICS 242
13.1.7.1 Business overview 242
13.1.7.2 Products offered 243
13.1.7.3 Recent developments 245
13.1.7.3.1 Product launches & approvals 245
13.1.7.3.2 Deals 246
13.1.8 MERCK KGAA 247
13.1.8.1 Business overview 247
13.1.8.2 Products offered 248
13.1.8.3 Recent developments 249
13.1.8.3.1 Deals 249
13.1.9 ACCELERATE DIAGNOSTICS, INC. 250
13.1.9.1 Business overview 250
13.1.9.2 Products offered 251
13.1.9.3 Recent developments 252
13.1.9.3.1 Product launches & approvals 252
13.1.9.3.2 Deals 252
13.1.9.3.3 Other developments 252
13.1.10 HIMEDIA LABORATORIES 253
13.1.10.1 Business overview 253
13.1.10.2 Products offered 254
13.1.11 LIOFILCHEM S.R.L. 255
13.1.11.1 Business overview 255
13.1.11.2 Products offered 255
13.1.12 ALIFAX S.R.L. 258
13.1.12.1 Business overview 258
13.1.12.2 Products offered 258
13.1.13 CREATIVE DIAGNOSTICS 259
13.1.13.1 Business overview 259
13.1.13.2 Products offered 260
13.1.14 SYNBIOSIS 261
13.1.14.1 Business overview 261
13.1.14.2 Products offered 262
13.1.15 BIOANALYSE 263
13.1.15.1 Business overview 263
13.1.15.2 Products offered 263
13.2 OTHER KEY PLAYERS 264
13.2.1 ZHUHAI DL BIOTECH CO., LTD. 264
13.2.2 ELITECHGROUP 265
13.2.3 MAST GROUP LTD. 267
13.2.4 CONDALAB 268
13.2.5 GENEFLUIDICS, INC. 269
13.2.6 BIOTRON HEALTHCARE 269
13.2.7 INVIVOGEN 270
13.2.8 MP BIOMEDICALS 271
13.2.9 QUANTAMATRIX INC. 271
13.2.10 SYSMEX EUROPE SE 272
13.2.11 COPAN DIAGNOSTICS INC. 272
13.2.12 ERBA DIAGNOSTICS MANNHEIM GMBH 273
14 APPENDIX 274
14.1 DISCUSSION GUIDE 274
14.2 KNOWLEDGESTORE: MARKETSANDMARKETS’ SUBSCRIPTION PORTAL 279
14.3 CUSTOMIZATION OPTIONS 281
14.4 RELATED REPORTS 281
14.5 AUTHOR DETAILS 282
❖ 世界の抗菌薬感受性試験市場に関するよくある質問(FAQ) ❖
・抗菌薬感受性試験の世界市場規模は?
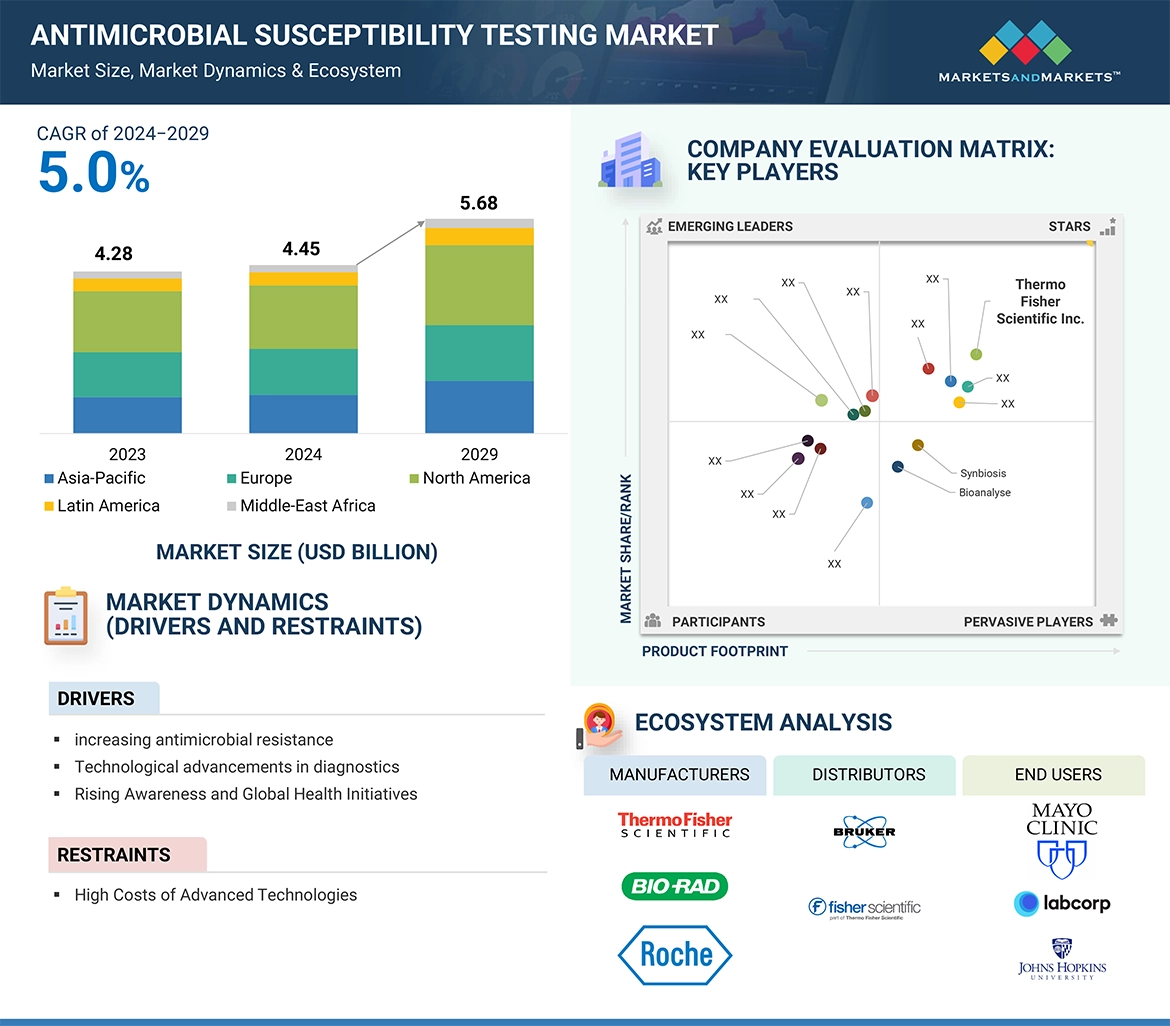
→MarketsandMarkets社は2024年の抗菌薬感受性試験の世界市場規模を44.5億米ドルと推定しています。
・抗菌薬感受性試験の世界市場予測は?
→MarketsandMarkets社は2029年の抗菌薬感受性試験の世界市場規模を56.8億米ドルと予測しています。
・抗菌薬感受性試験市場の成長率は?
→MarketsandMarkets社は抗菌薬感受性試験の世界市場が2024年~2029年に年平均5.0%成長すると予測しています。
・世界の抗菌薬感受性試験市場における主要企業は?
→MarketsandMarkets社は「BioMérieux (France)、Becton、Dickinson and company (US)、Thermo Fisher Scientific Inc. (US)、Danaher Corporation (US)、Bio-Rad laboratories (US)など ...」をグローバル抗菌薬感受性試験市場の主要企業として認識しています。
※上記FAQの市場規模、市場予測、成長率、主要企業に関する情報は本レポートの概要を作成した時点での情報であり、納品レポートの情報と少し異なる場合があります。