1 はじめに
1.1 調査目的 43
1.2 市場の定義 43
1.3 調査範囲 44
1.3.1 対象市場 44
1.3.2 対象範囲と除外範囲 45
1.3.3 考慮した年数 46
1.3.4 通貨 46
1.4 制限事項 46
1.5 利害関係者 46
1.6 変更点のまとめ 47
2 調査方法 48
2.1 調査データ 48
2.1.1 二次データ 49
2.1.2 一次データ 50
2.2 市場推計方法 51
2.2.1 市場推計 51
2.2.1.1 主要専門家の洞察 53
2.2.2 セグメント別の市場規模予測 54
2.3 市場成長率の予測 55
2.4 市場の内訳とデータの三角測量 57
2.5 リサーチの前提 58
2.6 調査の限界 58
2.7 リスク分析 59
3 エグゼクティブサマリー 60
4 プレミアムインサイト 66
4.1 遺伝子編集市場の概要 66
4.2 北米:遺伝子編集市場:タイプ別・国別(2023年) 67
4.3 遺伝子編集市場:地理的成長機会 67
5 市場の概要 68
5.1 はじめに 68
5.2 市場ダイナミクス 68
5.2.1 推進要因 69
5.2.1.1 個別化医療に対する需要の増加 69
5.2.1.2 遺伝子編集プロジェクトに対する有利な政府資金投資 71
5.2.1.3 遺伝子編集の応用拡大 72
5.2.2 阻害要因 72
5.2.2.1 オフターゲット効果と安全性の懸念 72
5.2.3 機会 73
5.2.3.1 プライム編集とブリッジRNAにおける技術進歩の高まり 73
5.2.3.2 先天性疾患の負担増加 73
5.2.4 課題 74
5.2.4.1 遺伝子編集ワークフローの拡張性に関する問題 74
5.2.4.2 高い設備・製造コスト 74
5.3 顧客のビジネスに影響を与えるトレンド/混乱 75
5.4 価格分析 76
5.4.1 主要プレイヤーの消耗品別平均販売価格動向(2023年) 76
5.4.2 遺伝子編集キット・試薬の平均コストレンジ(2021~2023年) 77
5.4.3 サービスの平均販売価格動向(タイプ別) 78
5.4.4 平均販売価格推移(地域別) 79
5.5 サプライチェーン分析 81
5.6 バリューチェーン分析 82
5.7 エコシステム分析 86
5.8 遺伝子編集市場におけるジェネレーティブAIの影響 87
5.9 技術分析 89
5.9.1 主要技術 89
5.9.1.1 次世代シーケンサー 89
5.9.1.2 エレクトロポレーション 89
5.9.1.3 マイクロインジェクション 89
5.9.2 補完的技術
5.9.2.1 合成生物学 90
5.9.2.2 データ解析 90
5.9.2.3 ハイスループット・スクリーニング 91
5.9.3 隣接技術 91
5.9.3.1 シングルセル解析 91
5.9.3.2 オプトジェネティクス 92
5.9.3.3 遺伝子導入システム 92
5.10 特許分析 92
5.11 主要会議・イベント(2024-2025年) 94
5.12 規制分析 95
5.12.1 規制の状況 95
5.12.1.1 北米 96
5.12.1.1.1 米国 96
5.12.1.1.2 カナダ 96
5.12.1.2 欧州 97
5.12.1.2.1 イギリス 97
5.12.1.3 アジア太平洋地域 98
5.12.1.3.1 中国 98
5.12.1.3.2 日本 98
5.12.1.3.3 韓国 98
5.12.1.3.4 オーストラリア 98
5.12.1.3.5 その他のアジア太平洋地域 99
5.12.2 規制機関、政府機関、その他の団体 100
5.13 ポーターの5つの力分析 102
5.13.1 新規参入の脅威 103
5.13.2 代替品の脅威 103
5.13.3 買い手の交渉力 103
5.13.4 供給者の交渉力 103
5.13.5 競合の激しさ 104
5.14 主要ステークホルダーと購買基準 104
5.14.1 購入プロセスにおける主要ステークホルダー 104
5.14.2 主要な購買基準 105
5.15 ケーススタディ分析 105
5.15.1 プライマリーヒトT細胞におけるハイブリッドSDNA HDRテンプレートを用いた遺伝子ノックイン効率の向上 105
5.15.2 Truecut cas9 protein v2およびTrueguide sgrnaを用いた癌細胞株およびT細胞における遺伝子編集効率 106
5.15.3 Puredit crispr cas9 rnpシステムの開発と特異性の最適化 106
5.15.4 効率的なin vitro遺伝子編集のためのsureguide cas9 programmable nuclease kitの評価 106
5.15.5 ジンクフィンガーヌクレアーゼ修飾造血幹細胞を用いた鎌状赤血球病の遺伝子編集 107
5.16 投資と資金調達のシナリオ 107
5.16.1 主な投資と資金調達活動(2021~2024年) 107
5.17 貿易データ分析 110
5.17.1 輸入データ(2019-2023年) 110
5.17.2 輸出データ、2019-2023 111
6 遺伝子編集市場、オファリング別 112
6.1 はじめに 113
6.2 製品 113
6.2.1 遺伝子改変技術の応用拡大が市場を牽引 113
市場を牽引 113
6.3 サービス 117
6.3.1 製薬会社による創薬開発のための高い取り込みが市場を促進 117
7 遺伝子編集製品市場:タイプ別 121
7.1 導入 122
7.2 試薬・消耗品 122
7.2.1 遺伝子編集キット 126
7.2.1.1 臨床現場における変異・スクリーニングキットの需要増が市場を牽引 126
市場を牽引 126
7.2.2 遺伝子編集ライブラリー 130
7.2.2.1 がん研究のための遺伝子スクリーニングが普及を促進 130
7.2.3 遺伝子編集試薬 133
7.2.3.1 動物モデル作成能力が市場成長を支える 133
7.3 ソフトウェアとシステム 137
7.3.1 クラウドベースの遺伝子編集プラットフォームの統合が市場を牽引 137
8 遺伝子編集試薬・消耗品市場(技術別) 141
8.1 イントロダクション 142
8.2 ノックアウト 142
8.2.1 crispr-cas9とtalensの改変が市場を牽引 142
8.3 ノックイン 146
8.3.1 ドナーDNAテンプレートとHDR経路の利用が市場を牽引 146
市場を牽引 146
8.4 遺伝子サイレンシング 149
8.4.1 rnai技術とアンチセンス療法の進歩が市場成長を支える 149
8.5 その他の技術 153
9 遺伝子編集製品市場(技術別) 156
9.1 導入 157
9.2 CRISPR 157
9.2.1 Cas9タンパク質の進歩が普及を後押し 157
9.3 TALENS 161
9.3.1 治療法開発の需要増加が市場を牽引 161
9.4 ZFN 164
9.4.1 カスタマイズされたデザインと標的編集の提供が
需要を後押し 164
9.5 塩基編集 167
9.5.1 機能ゲノミクスにおける用途の拡大が市場を後押し 167
9.6 アンチセンス 171
9.6.1 市場の成長を支えるオリゴヌクレオチド療法のイノベーション 171
9.7 RNAI 174
9.7.1 創薬への幅広い応用が市場拡大を促進 174
9.8 その他の技術 177
10 遺伝子編集製品市場:用途別 180
10.1 導入 181
10.2 細胞株工学 181
10.2.1 検証のための正確な遺伝子改変を提供する能力
市場を牽引する 181
10.3 ゲノム編集 185
10.3.1 バイオテクノロジーへの応用拡大が需要を押し上げる 185
10.4 創薬・医薬品開発 188
10.4.1 疾患プロファイリングのためのクリスプルを用いたシステムの普及が
市場を牽引
10.5 その他の用途 192
11 遺伝子編集製品市場(エンドユーザー別) 195
11.1 はじめに 196
11.2 製薬・バイオテクノロジー企業 196
11.2.1 増加する製品承認と強固な臨床パイプラインが市場を牽引 196
パイプラインが市場を牽引 196
11.3 学術・研究機関 200
11.3.1 ゲノムプロジェクトに対する有利な資金提供が市場を牽引 200
11.4 CROとCDMOS 203
11.4.1 臨床試験に特化した専門知識の提供が
市場を牽引 203
11.5 その他のエンドユーザー 206
12 遺伝子編集サービス市場:タイプ別 210
12.1 導入 211
12.2 細胞株開発&エンジニアリング 211
12.2.1 生物製剤とバイオシミラーの需要増加が市場を牽引 211
12.3 グルナ合成&ベクター構築 215
12.3.1 遺伝子編集技術の進歩と正確なターゲティングの需要が市場を牽引 215
12.4 ライブラリー構築とスクリーニング 218
12.4.1 ハイスループット・スクリーニングへの注目の高まりが市場を牽引 218
12.5 その他のサービス 222
12.5.1 その他のサービスにはオフターゲット解析や突然変異誘発サービスなど 222
13 遺伝子編集サービス市場:用途別 226
13.1 導入 227
13.2 細胞株工学 227
13.2.1 治療用バイオ生産のための研究開発における高い取り込みが市場を牽引 227
13.3 ゲノム編集 231
13.3.1 合成ゲノミクスへの関心の高まりが需要を押し上げる 231
13.4 創薬・医薬品開発 234
13.4.1 医薬品開発需要の高まりが市場拡大を促進 234
13.5 その他の用途 238
14 遺伝子編集サービス市場:エンドユーザー別 242
14.1 はじめに 243
14.2 製薬・バイオテクノロジー企業 243
14.2.1 先端モダリティに対する需要の高まりが市場を牽引 243
14.3 学術・研究機関 247
14.3.1 ゲノムプロジェクトに対する資金提供の増加が市場を牽引 247
14.4 その他のエンドユーザー 250
15 遺伝子編集市場:地域別 254
15.1 はじめに 255
15.2 北米 256
15.2.1 北米のマクロ経済見通し 261
15.2.2 米国 262
15.2.2.1 政府当局と民間企業による投資の増加が市場を牽引 262
15.2.3 カナダ 266
15.2.3.1 ゲノミクスとバイオテクノロジーの研究開発イニシアチブの増加が成長を促進 266
15.3 欧州 271
15.3.1 欧州のマクロ経済見通し 276
15.3.2 ドイツ 278
15.3.2.1 バイオテクノロジー分野の技術革新促進に向けた注目の高まりが市場を後押し 278
15.3.3 フランス 283
15.3.3.1 進行中の臨床試験と先進的ゲノム研究が成長を促進 283
成長を促進 283
15.3.4 イギリス 287
15.3.4.1 細胞・遺伝子治療インフラ整備への注目の高まりが市場を後押し 287
15.3.5 イタリア 292
15.3.5.1 成長を促進する研究資金の増加 292
15.3.6 スペイン 296
15.3.6.1 活況を呈するバイオテクノロジー分野が市場成長に寄与 296
15.3.7 その他の欧州 301
15.4 アジア太平洋地域 306
15.4.1 アジア太平洋地域のマクロ経済見通し 311
15.4.2 中国 312
15.4.2.1 手頃な価格のシーケンシングプラットフォームと手技の増加が成長を後押し 312
15.4.3 日本 316
15.4.3.1 がん研究への関心の高まりが市場を後押し 316
15.4.4 インド 321
15.4.4.1 ゲノム工学の治療効果に対する意識の高まりが成長を後押し 321
15.4.5 韓国 326
15.4.5.1 in vivo遺伝子編集への志向の高まりが成長を刺激 326
15.4.6 オーストラリア 330
15.4.6.1 好ましい規制環境が成長を持続 330
15.4.7 その他のアジア太平洋地域 335
15.5 ラテンアメリカ 339
15.5.1 ラテンアメリカのマクロ経済見通し 344
15.5.2 ブラジル 345
15.5.2.1 バイオテクノロジー技術革新を支援する規制的枠組みが市場を活性化 345
15.5.3 その他のラテンアメリカ 349
15.6 中東 353
15.6.1 中東のマクロ経済見通し 354
15.6.2 GCC諸国 359
15.6.2.1 サウジアラビア王国 364
15.6.2.1.1 市場成長を支えるバイオベンチャー企業の設立増加 364
市場の成長を支える 364
15.6.2.2 アラブ首長国連邦(UAE) 368
15.6.2.2.1 ゲノム配列決定のための共同研究の増加が市場を牽引 368
市場を牽引 368
15.6.2.3 その他のGCC諸国 373
15.6.3 その他の中東地域 377
15.7 アフリカ 382
15.7.1 医療インフラの改善が市場成長を支える 382
15.7.2 アフリカのマクロ経済見通し 386
16 競争環境 388
16.1 はじめに 388
16.2 主要プレーヤーの戦略/勝利への権利(2021-2024年) 388
16.3 収益分析、2019-2023年 392
16.4 市場シェア分析、2023年 394
16.4.1 主要市場プレーヤーのランキング 395
16.5 企業評価と財務指標 396
16.5.1 企業評価 396
16.5.2 財務指標 396
16.6 ブランド/製品の比較 397
16.6.1 サーモフィッシャーサイエンティフィック(米国) 397
397 16.6.2 メルクKGAA(ドイツ
16.6.3 ジェンスクリプト(米国) 398
16.6.4 アジレント・テクノロジー(米国) 398
16.6.5 レヴィティ(米国) 398
16.7 企業評価マトリックス:主要企業(2023年) 398
16.7.1 スター企業 398
16.7.2 新興リーダー 398
16.7.3 浸透型プレーヤー 398
16.7.4 参加企業 399
16.7.5 企業フットプリント:主要プレイヤー(2023年) 400
16.7.5.1 企業フットプリント 400
16.7.5.2 製品フットプリント 401
16.7.5.3 サービスのフットプリント 402
16.7.5.4 技術のフットプリント 403
16.7.5.5 地域別フットプリント 405
16.8 企業評価マトリクス:新興企業/SM(2023年) 406
16.8.1 進歩的企業 406
16.8.2 対応力のある企業 406
16.8.3 ダイナミックな企業 406
16.8.4 スタートアップ・ブロック 406
16.8.5 競争ベンチマーキング:新興企業/SM(2023年) 408
16.8.5.1 主要新興企業/中小企業の詳細リスト 408
16.8.5.2 主要新興企業/中小企業の競争ベンチマーク 409
16.9 競争シナリオ 409
16.9.1 製品/サービスの上市と承認 410
16.9.2 取引 411
16.9.3 拡張 413
17 企業プロフィール 414
Thermo Fisher Scientific Inc. (US)
Merck KGaA (Germany)
GenScript (US)
Agilent Technologies Inc. (US)
Revvity (US)
Lonza (US)
Tecan Trading AG (Switzerland)
Sangamo therapeutics (US). Precision BioSciences (US)
Cellectis S.A. (France)
Regeneron Pharmaceuticals Inc. (US)
AMSBIO (UK)
Creative Biogene (US)
Synthego (US)
Takara Bio Inc. (Japan)
Bio-Techne (US)
Caribou Biosciences Inc. (US) Bioneer corporation (South Korea) and REPROCELL Inc. (Japan).
18 付録 489
18.1 ディスカッションガイド 489
18.2 Knowledgestore: Marketsandmarketsの購読ポータル 494
18.3 カスタマイズオプション 496
18.4 関連レポート 496
18.5 著者の詳細 497
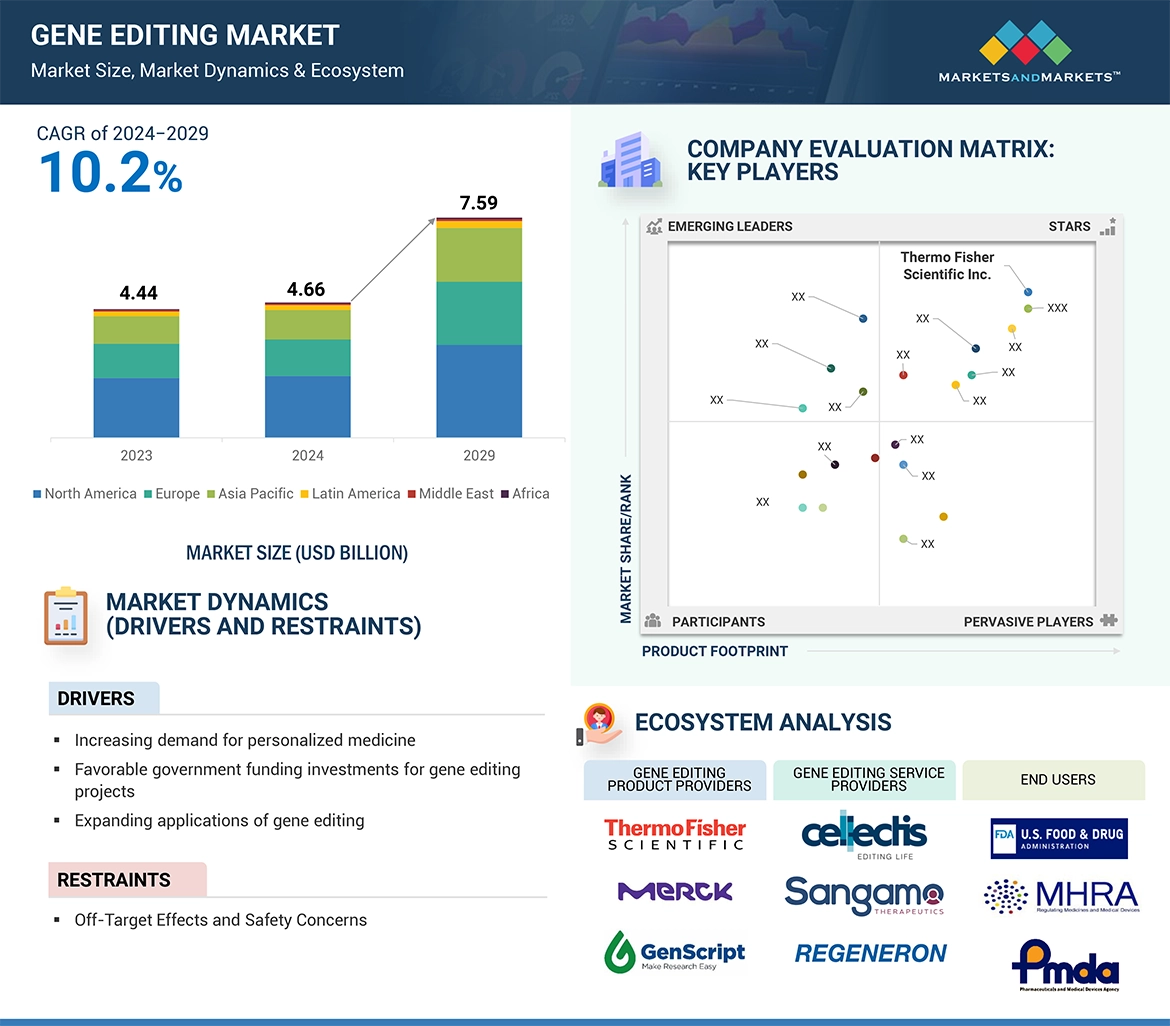
Factors such as advancements in gene editing technologies, and the expanding range of gene editing applications are driving the growth of the gene editing market. However, the market faces several challenges due to off-target effects of gene editing, along with difficulties in scalability and manufacturing, pose significant hurdles.
“The product segment accounted for the largest share by offerings segment in the gene editing market in 2023”
By offerings, the gene editing market is segmented into products and services. The products segment held the largest share in 2023. The demand for products in gene editing process including experimentation and research for cell line development and drug development & discovery make them an indispensable part of the gene market. Gene editing products as reagents & consumables and Software & systems like gene editing kits, gene editing reagents and gene editing libraries are utilized by pharmaceutican & biotechnology companies and academic & research institutes in various gene editing workflow. Services held the second highest share of the gene editing market in 2023.
“The CRISPR technology held the highest market share of the gene editing products market by technology in 2023”
By Technology, the gene editing products market is segmented into CRISPR, TALEN, ZFN, Base editing, Antisense, RNAi and other technologies which include piggybac and prime editing among others. The CRISPR segment held the highest share of the gene editing products by technology market in 2023 driven by advancements in CRISPR technology leading to large scale adoption of this technology in gene editing. Gene editing products are used in a large range of CRISPR applications including reagents like Cas-9, vectors, libraries for screening and target identification and CRISPR based gene editing kits. Still, TALEN segment held the second largest share of the market in 2023.
“The Asia Pacific region is growing at the highest CAGR in the gene editing market from 2024 to 2029.”
The Asia Pacific is estimated to be the fastest-growing segment of the market owing to the a significant focus on R&D activities, funding by government and private players in gene editing research, and rising adoption of gene editing technologies in drug discovery & development. Further, the rising prevalence of rare and genetic diseases is attributed to the aging population of countries such as Japan, and China. This is supporting the adoption of gene editing technologies in healthcare management and treatment driving the market growth. However, North America held the largest share of gene editing market in 2023.
The primary interviews conducted for this report can be categorized as follows:
• By Respondent: Supply Side- 70% and Demand Side 30%
• By Designation: Managers - 45% CXOs and Director-level - 30%, and Executives - 25%,
• By Region: North America -40%, Europe -25%, Asia-Pacific -25%, Latin America -5%, and Middle East -5%.
List of Companies Profiled in the Report:
• Thermo fisher scientific Inc. (US)
• Merck KGaA (Germany)
• GenScript (US)
• Agilent Technologies, Inc. (US)
• Revvity (US)
• Lonza (US)
• Tecan Trading AG (Switzerland)
• Sangamo therapeutics (US)
• Precision BioSciences (US)
• Cellectis S.A. (France)
• Regeneron Pharmaceuticals Inc. (US)
• AMSBIO (UK)
• Creative Biogene (US)
• Synthego (US)
• Takara Bio Inc. (Japan)
• Bio-Techne (US)
• Caribou Biosciences, Inc. (US)
• Bioneer corporation (South Korea)
• REPROCELL Inc. (Japan)
Research Coverage:
This research report categorizes the gene editing market by offerings (products and services), products by type (reagnets & consumables, and software & systems), reagents & consumables by Technique (knock-out, knock-in, gene silencing, and other techniques), products by technology (CRISPR, TALEN, ZFN, Base editing, Antisense, RNAi and other technologies), products by application (cell line engineering, genome editing/genetic engineering, drug discovery & development, and other applications), products by end user (pharmaceutical & biotechnology companies, academic & research institutes, CROs & CDMOs, and other end users), services by type (cell line engineering, gRNA synthesis & vector construction, library construction & screening, and other types), services by application (cell line development & engineering, genome editing/genetic engineering, drug discovery & development, and other applications), services by end user (pharmaceutical & biotechnology companies, academic & research institutes, and other end users) and by region (North America, Europe, Asia Pacific, Latin America, Middle East, and Africa). The scope of the report covers detailed information regarding the major factors, such as drivers, challenges, opportunities, and restraints influencing the growth of the genomics market. A detailed analysis of the key industry players has been done to provide insights into their business overview, product & service portfolio, key strategies such as product & service launches, collaborations, partnerships, expansions, agreements, and recent developments associated with the gene editing market. Competitive analysis of top players and upcoming startups in the gene editing market ecosystem is covered in this report.
Key Benefits of Buying the Report:
The report will help market leaders/new entrants by providing them with the closest approximations of the revenue numbers for the overall gene editing market and its subsegments. It will also help stakeholders better understand the competitive landscape and gain more insights to better position their business and make suitable go-to-market strategies. This report will enable stakeholders to understand the market's pulse and provide them with information on the key market drivers, restraints, opportunities, and challenges.
The report provides insights on the following pointers:
Analysis of key drivers (Increasing demand for personalized medicine, Favorable government funding investments for gene editing projects, Expanding applications of gene editing), restraints (Off-Target Effects and Safety Concerns), opportunities (Technological advancements in Prime editing and bridge RNA, Rising burden of congenital disorders), and challenges (Issues with scalability of gene editing workflows. High equipment and facility costs) influencing the growth of the market.
• Product Development/Innovation: Detailed insights on newly launched product/services of the gene editing market
• Market Development: Comprehensive information about lucrative markets - the report analyses the market across varied regions.
• Market Diversification: Exhaustive information about new products & services, untapped geographies, recent developments, and investments in the gene editing market
• Competitive Assessment: Thermo Fisher Scientific Inc. (US), Merck KGaA (Germany), GenScript (US), Agilent Technologies, Inc. (US), Revvity (US), Lonza (US), Tecan Trading AG (Switzerland), Sangamo therapeutics (US). Precision BioSciences (US), Cellectis S.A. (France), Regeneron Pharmaceuticals Inc. (US), AMSBIO (UK), Creative Biogene (US), Synthego (US), Takara Bio Inc. (Japan), Bio-Techne (US), Caribou Biosciences, Inc. (US) Bioneer corporation (South Korea), and REPROCELL Inc. (Japan).
1 INTRODUCTION 43
1.1 STUDY OBJECTIVES 43
1.2 MARKET DEFINITION 43
1.3 STUDY SCOPE 44
1.3.1 MARKETS COVERED 44
1.3.2 INCLUSIONS & EXCLUSIONS 45
1.3.3 YEARS CONSIDERED 46
1.3.4 CURRENCY CONSIDERED 46
1.4 LIMITATIONS 46
1.5 STAKEHOLDERS 46
1.6 SUMMARY OF CHANGES 47
2 RESEARCH METHODOLOGY 48
2.1 RESEARCH DATA 48
2.1.1 SECONDARY DATA 49
2.1.2 PRIMARY DATA 50
2.2 MARKET ESTIMATION METHODOLOGY 51
2.2.1 MARKET ESTIMATION 51
2.2.1.1 Insights of primary experts 53
2.2.2 SEGMENTAL MARKET SIZE ESTIMATIONS 54
2.3 MARKET GROWTH RATE PROJECTIONS 55
2.4 MARKET BREAKDOWN AND DATA TRIANGULATION 57
2.5 RESEARCH ASSUMPTIONS 58
2.6 RESEARCH LIMITATIONS 58
2.7 RISK ANALYSIS 59
3 EXECUTIVE SUMMARY 60
4 PREMIUM INSIGHTS 66
4.1 GENE EDITING MARKET OVERVIEW 66
4.2 NORTH AMERICA: GENE EDITING MARKET, BY TYPE AND COUNTRY (2023) 67
4.3 GENE EDITING MARKET: GEOGRAPHIC GROWTH OPPORTUNITIES 67
5 MARKET OVERVIEW 68
5.1 INTRODUCTION 68
5.2 MARKET DYNAMICS 68
5.2.1 DRIVERS 69
5.2.1.1 Increasing demand for personalized medicine 69
5.2.1.2 Favorable government funding investments for gene editing projects 71
5.2.1.3 Expanding applications of gene editing 72
5.2.2 RESTRAINTS 72
5.2.2.1 Off-target effects and safety concerns 72
5.2.3 OPPORTUNITIES 73
5.2.3.1 Rising technological advancements in prime editing and bridge RNA 73
5.2.3.2 Increasing burden of congenital disorders 73
5.2.4 CHALLENGES 74
5.2.4.1 Issues associated with scalability of gene editing workflows 74
5.2.4.2 High equipment & manufacturing costs 74
5.3 TRENDS/DISRUPTIONS IMPACTING CUSTOMERS’ BUSINESSES 75
5.4 PRICING ANALYSIS 76
5.4.1 AVERAGE SELLING PRICE TREND OF KEY PLAYERS, BY CONSUMABLE (2023) 76
5.4.2 AVERAGE COST RANGE OF GENE EDITING KITS & REAGENTS, 2021−2023 77
5.4.3 AVERAGE SELLING PRICE TREND OF SERVICES, BY TYPE 78
5.4.4 AVERAGE SELLING PRICE, BY REGION 79
5.5 SUPPLY CHAIN ANALYSIS 81
5.6 VALUE CHAIN ANALYSIS 82
5.7 ECOSYSTEM ANALYSIS 86
5.8 IMPACT OF GENERATIVE AI ON GENE EDITING MARKET 87
5.9 TECHNOLOGY ANALYSIS 89
5.9.1 KEY TECHNOLOGIES 89
5.9.1.1 Next-generation sequencing 89
5.9.1.2 Electroporation 89
5.9.1.3 Microinjection 89
5.9.2 COMPLEMENTARY TECHNOLOGIES 90
5.9.2.1 Synthetic biology 90
5.9.2.2 Data analysis 90
5.9.2.3 High-throughput screening 91
5.9.3 ADJACENT TECHNOLOGIES 91
5.9.3.1 Single-cell analysis 91
5.9.3.2 Optogenetics 92
5.9.3.3 Gene delivery systems 92
5.10 PATENT ANALYSIS 92
5.11 KEY CONFERENCES AND EVENTS, 2024−2025 94
5.12 REGULATORY ANALYSIS 95
5.12.1 REGULATORY LANDSCAPE 95
5.12.1.1 North America 96
5.12.1.1.1 US 96
5.12.1.1.2 Canada 96
5.12.1.2 Europe 97
5.12.1.2.1 UK 97
5.12.1.3 Asia Pacific 98
5.12.1.3.1 China 98
5.12.1.3.2 Japan 98
5.12.1.3.3 South Korea 98
5.12.1.3.4 Australia 99
5.12.1.3.5 Rest of Asia Pacific 99
5.12.2 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS 100
5.13 PORTER’S FIVE FORCES ANALYSIS 102
5.13.1 THREAT OF NEW ENTRANTS 103
5.13.2 THREAT OF SUBSTITUTES 103
5.13.3 BARGAINING POWER OF BUYERS 103
5.13.4 BARGAINING POWER OF SUPPLIERS 103
5.13.5 INTENSITY OF COMPETITIVE RIVALRY 104
5.14 KEY STAKEHOLDERS AND BUYING CRITERIA 104
5.14.1 KEY STAKEHOLDERS IN BUYING PROCESS 104
5.14.2 KEY BUYING CRITERIA 105
5.15 CASE STUDY ANALYSIS 105
5.15.1 ENHANCED GENE KNOCK-IN EFFICIENCY USING HYBRID SSDNA HDR TEMPLATES IN PRIMARY HUMAN T-CELLS 105
5.15.2 GENE EDITING EFFICIENCY IN CANCER CELL LINES AND T-CELLS USING TRUECUT CAS9 PROTEIN V2 AND TRUEGUIDE SGRNA 106
5.15.3 DEVELOPMENT & SPECIFICITY OPTIMIZATION OF PUREDIT CRISPR CAS9 RNP SYSTEM 106
5.15.4 EVALUATION OF SUREGUIDE CAS9 PROGRAMMABLE NUCLEASE KIT FOR EFFICIENT IN VITRO GENE EDITING 106
5.15.5 GENE EDITING FOR SICKLE-CELL DISEASE USING ZINC FINGER NUCLEASE-MODIFIED HEMATOPOIETIC STEM CELLS 107
5.16 INVESTMENT & FUNDING SCENARIO 107
5.16.1 KEY INVESTMENTS & FUNDING ACTIVITIES, 2021−2024 107
5.17 TRADE DATA ANALYSIS 110
5.17.1 IMPORT DATA, 2019−2023 110
5.17.2 EXPORT DATA, 2019−2023 111
6 GENE EDITING MARKET, BY OFFERING 112
6.1 INTRODUCTION 113
6.2 PRODUCTS 113
6.2.1 EXPANDING APPLICATIONS OF GENE MODIFICATION TECHNIQUES TO
DRIVE MARKET 113
6.3 SERVICES 117
6.3.1 HIGH UPTAKE BY PHARMA COMPANIES FOR DRUG DISCOVERY & DEVELOPMENT TO PROPEL MARKET 117
7 GENE EDITING PRODUCTS MARKET, BY TYPE 121
7.1 INTRODUCTION 122
7.2 REAGENTS & CONSUMABLES 122
7.2.1 GENE EDITING KITS 126
7.2.1.1 Increasing demand for mutation & screening kits in
clinical settings to propel market 126
7.2.2 GENE EDITING LIBRARIES 130
7.2.2.1 Genetic screening for cancer research to fuel uptake 130
7.2.3 GENE EDITING REAGENTS 133
7.2.3.1 Ability to create animal models to support market growth 133
7.3 SOFTWARE & SYSTEMS 137
7.3.1 INTEGRATION OF CLOUD-BASED PLATFORMS FOR NGS TO DRIVE MARKET 137
8 GENE EDITING REAGENTS & CONSUMABLES MARKET, BY TECHNIQUE 141
8.1 INTRODUCTION 142
8.2 KNOCK-OUT 142
8.2.1 MODIFICATION OF CRISPR-CAS9 AND TALENS TO PROPEL MARKET 142
8.3 KNOCK-IN 146
8.3.1 UTILIZATION OF DONOR DNA TEMPLATES AND HDR PATHWAYS TO
DRIVE MARKET 146
8.4 GENE SILENCING 149
8.4.1 ADVANCEMENTS IN RNAI TECHNOLOGY AND ANTISENSE THERAPY TO SUPPORT MARKET GROWTH 149
8.5 OTHER TECHNIQUES 153
9 GENE EDITING PRODUCTS MARKET, BY TECHNOLOGY 156
9.1 INTRODUCTION 157
9.2 CRISPR 157
9.2.1 ADVANCEMENTS IN CAS9 PROTEINS TO FUEL UPTAKE 157
9.3 TALENS 161
9.3.1 INCREASING DEMAND FOR THERAPEUTIC DEVELOPMENT TO DRIVE MARKET 161
9.4 ZFN 164
9.4.1 PROVISION OF CUSTOMIZED DESIGN AND TARGETED EDITS TO
BOOST DEMAND 164
9.5 BASE EDITING 167
9.5.1 GROWING APPLICATIONS IN FUNCTIONAL GENOMICS TO FUEL MARKET 167
9.6 ANTISENSE 171
9.6.1 INNOVATIONS IN OLIGONUCLEOTIDE THERAPIES TO SUPPORT MARKET GROWTH 171
9.7 RNAI 174
9.7.1 WIDE APPLICATIONS IN DRUG DISCOVERY TO FUEL UPTAKE 174
9.8 OTHER TECHNOLOGIES 177
10 GENE EDITING PRODUCTS MARKET, BY APPLICATION 180
10.1 INTRODUCTION 181
10.2 CELL LINE ENGINEERING 181
10.2.1 ABILITY TO PROVIDE PRECISE GENE MODIFICATIONS FOR VALIDATION
TO DRIVE MARKET 181
10.3 GENOME EDITING 185
10.3.1 GROWING APPLICATIONS IN BIOTECHNOLOGY TO BOOST DEMAND 185
10.4 DRUG DISCOVERY & DEVELOPMENT 188
10.4.1 UPTAKE OF CRISPR-BASED SYSTEMS FOR DISEASE PROFILING TO
DRIVE MARKET 188
10.5 OTHER APPLICATIONS 192
11 GENE EDITING PRODUCTS MARKET, BY END USER 195
11.1 INTRODUCTION 196
11.2 PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES 196
11.2.1 INCREASING PRODUCT APPROVALS AND ROBUST CLINICAL
PIPELINE TO PROPEL MARKET 196
11.3 ACADEMIC & RESEARCH INSTITUTES 200
11.3.1 FAVORABLE FUNDING FOR GENOMIC PROJECTS TO DRIVE MARKET 200
11.4 CROS & CDMOS 203
11.4.1 PROVISION OF SPECIALIZED EXPERTISE FOR CLINICAL TRIALS TO
DRIVE MARKET 203
11.5 OTHER END USERS 206
12 GENE EDITING SERVICES MARKET, BY TYPE 210
12.1 INTRODUCTION 211
12.2 CELL LINE DEVELOPMENT & ENGINEERING 211
12.2.1 GROWING DEMAND FOR BIOLOGICS AND BIOSIMILARS TO DRIVE MARKET 211
12.3 GRNA SYNTHESIS & VECTOR CONSTRUCTION 215
12.3.1 ADVANCEMENTS IN GENE EDITING TECHNOLOGIES AND DEMAND FOR PRECISE TARGETING TO DRIVE MARKET 215
12.4 LIBRARY CONSTRUCTION & SCREENING 218
12.4.1 INCREASING FOCUS ON HIGH-THROUGHPUT SCREENING TO PROPEL MARKET 218
12.5 OTHER SERVICES 222
12.5.1 OTHER SERVICES INCLUDE OFF-TARGET ANALYSIS AND MUTAGENESIS SERVICES. 222
13 GENE EDITING SERVICES MARKET, BY APPLICATION 226
13.1 INTRODUCTION 227
13.2 CELL LINE ENGINEERING 227
13.2.1 HIGH UPTAKE IN R&D FOR THERAPEUTIC BIOPRODUCTION TO DRIVE MARKET 227
13.3 GENOME EDITING 231
13.3.1 GROWING FOCUS ON SYNTHETIC GENOMICS TO BOOST DEMAND 231
13.4 DRUG DISCOVERY & DEVELOPMENT 234
13.4.1 GROWING DEMAND FOR PHARMACEUTICAL DEVELOPMENT TO FUEL UPTAKE 234
13.5 OTHER APPLICATIONS 238
14 GENE EDITING SERVICES MARKET, BY END USER 242
14.1 INTRODUCTION 243
14.2 PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES 243
14.2.1 GROWING DEMAND FOR ADVANCED MODALITIES TO DRIVE MARKET 243
14.3 ACADEMIC & RESEARCH INSTITUTES 247
14.3.1 INCREASING FUNDING FOR GENOMIC PROJECTS TO PROPEL MARKET 247
14.4 OTHER END USERS 250
15 GENE EDITING MARKET, BY REGION 254
15.1 INTRODUCTION 255
15.2 NORTH AMERICA 256
15.2.1 MACROECONOMIC OUTLOOK FOR NORTH AMERICA 261
15.2.2 US 262
15.2.2.1 Growing investments by government authorities and private players to drive market 262
15.2.3 CANADA 266
15.2.3.1 Rise in research & development initiatives for genomics and biotechnology to augment growth 266
15.3 EUROPE 271
15.3.1 MACROECONOMIC OUTLOOK FOR EUROPE 276
15.3.2 GERMANY 278
15.3.2.1 Growing focus on promoting innovations in biotechnology sector to boost market 278
15.3.3 FRANCE 283
15.3.3.1 Ongoing clinical trials and advanced genomic research to
promote growth 283
15.3.4 UK 287
15.3.4.1 Growing focus on developing cell and gene therapy infrastructure to fuel market 287
15.3.5 ITALY 292
15.3.5.1 Increasing availability of research funds to expedite growth 292
15.3.6 SPAIN 296
15.3.6.1 Booming biotechnology sector to contribute to market growth 296
15.3.7 REST OF EUROPE 301
15.4 ASIA PACIFIC 306
15.4.1 MACROECONOMIC OUTLOOK FOR ASIA PACIFIC 311
15.4.2 CHINA 312
15.4.2.1 Increasing affordability of sequencing platforms and procedures to favor growth 312
15.4.3 JAPAN 316
15.4.3.1 Growing focus on cancer research to fuel market 316
15.4.4 INDIA 321
15.4.4.1 Increased awareness about therapeutic benefits of genome engineering to encourage growth 321
15.4.5 SOUTH KOREA 326
15.4.5.1 Rising inclination toward in vivo gene editing to stimulate growth 326
15.4.6 AUSTRALIA 330
15.4.6.1 Favorable regulatory landscape to sustain growth 330
15.4.7 REST OF ASIA PACIFIC 335
15.5 LATIN AMERICA 339
15.5.1 MACROECONOMIC OUTLOOK FOR LATIN AMERICA 344
15.5.2 BRAZIL 345
15.5.2.1 Supportive regulatory framework for biotechnology innovation to fuel market 345
15.5.3 REST OF LATIN AMERICA 349
15.6 MIDDLE EAST 353
15.6.1 MACROECONOMIC OUTLOOK FOR MIDDLE EAST 354
15.6.2 GCC COUNTRIES 359
15.6.2.1 Kingdom of Saudi Arabia 364
15.6.2.1.1 Increasing establishment of biotech startups to
support market growth 364
15.6.2.2 United Arab Emirates (UAE) 368
15.6.2.2.1 Growing collaborations for genome sequencing to
drive market 368
15.6.2.3 Other GCC Countries 373
15.6.3 REST OF MIDDLE EAST 377
15.7 AFRICA 382
15.7.1 IMPROVEMENTS IN HEALTHCARE INFRASTRUCTURE TO SUPPORT MARKET GROWTH 382
15.7.2 MACROECONOMIC OUTLOOK FOR AFRICA 386
16 COMPETITIVE LANDSCAPE 388
16.1 INTRODUCTION 388
16.2 KEY PLAYER STRATEGIES/RIGHT TO WIN, 2021−2024 388
16.3 REVENUE ANALYSIS, 2019−2023 392
16.4 MARKET SHARE ANALYSIS, 2023 394
16.4.1 RANKING OF KEY MARKET PLAYERS 395
16.5 COMPANY VALUATION AND FINANCIAL METRICS 396
16.5.1 COMPANY VALUATION 396
16.5.2 FINANCIAL METRICS 396
16.6 BRAND/PRODUCT COMPARISON 397
16.6.1 THERMO FISHER SCIENTIFIC INC. (US) 397
16.6.2 MERCK KGAA (GERMANY) 397
16.6.3 GENSCRIPT (US) 398
16.6.4 AGILENT TECHNOLOGIES, INC. (US) 398
16.6.5 REVVITY, INC. (US) 398
16.7 COMPANY EVALUATION MATRIX: KEY PLAYERS, 2023 398
16.7.1 STARS 398
16.7.2 EMERGING LEADERS 398
16.7.3 PERVASIVE PLAYERS 398
16.7.4 PARTICIPANTS 399
16.7.5 COMPANY FOOTPRINT: KEY PLAYERS, 2023 400
16.7.5.1 Company footprint 400
16.7.5.2 Product footprint 401
16.7.5.3 Service footprint 402
16.7.5.4 Technology footprint 403
16.7.5.5 Region footprint 405
16.8 COMPANY EVALUATION MATRIX: STARTUPS/SMES, 2023 406
16.8.1 PROGRESSIVE COMPANIES 406
16.8.2 RESPONSIVE COMPANIES 406
16.8.3 DYNAMIC COMPANIES 406
16.8.4 STARTING BLOCKS 406
16.8.5 COMPETITIVE BENCHMARKING: STARTUPS/SMES, 2023 408
16.8.5.1 Detailed list of key startups/SMEs 408
16.8.5.2 Competitive benchmarking of key startups/SMEs 409
16.9 COMPETITIVE SCENARIO 409
16.9.1 PRODUCT/SERVICE LAUNCHES & APPROVALS 410
16.9.2 DEALS 411
16.9.3 EXPANSIONS 413
17 COMPANY PROFILES 414
17.1 KEY PLAYERS 414
17.1.1 THERMO FISHER SCIENTIFIC INC. 414
17.1.1.1 Business overview 414
17.1.1.2 Products/Services offered 415
17.1.1.3 Recent developments 417
17.1.1.3.1 Product/Service launches & approvals 417
17.1.1.3.2 Deals 417
17.1.1.3.3 Expansions 418
17.1.1.4 MnM view 418
17.1.1.4.1 Key strengths 418
17.1.1.4.2 Strategic choices 418
17.1.1.4.3 Weaknesses & competitive threats 418
17.1.2 MERCK KGAA 419
17.1.2.1 Business overview 419
17.1.2.2 Products/Services offered 420
17.1.2.3 Recent developments 422
17.1.2.3.1 Deals 422
17.1.2.4 MnM view 422
17.1.2.4.1 Key strengths 422
17.1.2.4.2 Strategic choices 422
17.1.2.4.3 Weaknesses & competitive threats 423
17.1.3 GENSCRIPT 424
17.1.3.1 Business overview 424
17.1.3.2 Products/Services offered 425
17.1.3.3 Recent developments 427
17.1.3.3.1 Product/Service launches & approvals 427
17.1.3.3.2 Deals 428
17.1.3.3.3 Expansions 428
17.1.3.4 MnM view 428
17.1.3.4.1 Key strengths 428
17.1.3.4.2 Strategic choices 429
17.1.3.4.3 Weaknesses and competitive threats 429
17.1.4 AGILENT TECHNOLOGIES, INC. 430
17.1.4.1 Business overview 430
17.1.4.2 Products/Services offered 431
17.1.4.3 Recent developments 433
17.1.4.3.1 Deals 433
17.1.4.3.2 Expansions 434
17.1.4.4 MnM view 434
17.1.4.4.1 Key strengths 434
17.1.4.4.2 Strategic choices 434
17.1.4.4.3 Weaknesses & competitive threats 434
17.1.5 REVVITY, INC. 435
17.1.5.1 Business overview 435
17.1.5.2 Products/Services offered 436
17.1.5.3 Recent developments 439
17.1.5.3.1 Product/Service launches 439
17.1.5.3.2 Deals 439
17.1.5.4 MnM view 440
17.1.5.4.1 Key strengths 440
17.1.5.4.2 Strategic choices 440
17.1.5.4.3 Weakness & competitive threats 440
17.1.6 LONZA 441
17.1.6.1 Business overview 441
17.1.6.2 Products/Services offered 442
17.1.6.3 Recent developments 443
17.1.6.3.1 Product/Service launches 443
17.1.7 TECAN TRADING AG 444
17.1.7.1 Business overview 444
17.1.7.2 Products/Services offered 445
17.1.7.3 Recent developments 446
17.1.7.3.1 Deals 446
17.1.8 SANGAMO THERAPEUTICS 447
17.1.8.1 Business overview 447
17.1.8.2 Products/Services offered 448
17.1.8.3 Recent developments 448
17.1.8.3.1 Deals 448
17.1.9 PRECISION BIOSCIENCES 449
17.1.9.1 Business overview 449
17.1.9.2 Products/Services offered 450
17.1.9.3 Recent developments 450
17.1.9.3.1 Deals 450
17.1.10 CELLECTIS S.A. 451
17.1.10.1 Business overview 451
17.1.10.2 Products/Services offered 452
17.1.10.3 Recent developments 452
17.1.10.3.1 Deals 452
17.1.11 REGENERON PHARMACEUTICALS, INC. 453
17.1.11.1 Business overview 453
17.1.11.2 Products/Services offered 454
17.1.11.3 Recent developments 454
17.1.11.3.1 Deals 454
17.1.12 AMSBIO 455
17.1.12.1 Business overview 455
17.1.12.2 Products/Services offered 455
17.1.13 CREATIVE BIOGENE 457
17.1.13.1 Business overview 457
17.1.13.2 Products/Services offered 457
17.1.14 SYNTHEGO 460
17.1.14.1 Business overview 460
17.1.14.2 Products/Services offered 460
17.1.14.3 Recent developments 462
17.1.14.3.1 Product/Service launches 462
17.1.14.3.2 Deals 462
17.1.14.3.3 Expansions 462
17.1.15 TAKARA BIO INC. 463
17.1.15.1 Business overview 463
17.1.15.2 Products/Services offered 464
17.1.15.3 Recent developments 466
17.1.15.3.1 Deals 466
17.1.15.3.2 Expansions 466
17.1.16 BIO-TECHNE 467
17.1.16.1 Business overview 467
17.1.16.2 Products/Services offered 468
17.1.16.3 Recent developments 470
17.1.16.3.1 Deals 470
17.1.16.3.2 Expansions 470
17.1.17 CARIBOU BIOSCIENCES, INC. 471
17.1.17.1 Business overview 471
17.1.17.2 Products/Services offered 472
17.1.17.3 Recent developments 472
17.1.17.3.1 Deals 472
17.1.18 BIONEER CORPORATION 473
17.1.18.1 Business overview 473
17.1.18.2 Products/Services offered 474
17.1.19 REPROCELL INC. 475
17.1.19.1 Business overview 475
17.1.19.2 Products/Services offered 476
17.2 OTHER PLAYERS 477
17.2.1 ALSTEM INC. 477
17.2.2 NEW ENGLAND BIOLABS 478
17.2.3 INSCRIPTA, INC. 479
17.2.4 BIOCAT GMBH. 480
17.2.5 INTEGRATED DNA TECHNOLOGIES, INC. 481
17.2.6 COBO TECHNOLOGIES APS 482
17.2.7 GENECOPOEIA, INC. 483
17.2.8 HERA BIOLABS 484
17.2.9 STEMCELL TECHNOLOGIES. 485
17.2.10 AXOL BIOSCIENCE LTD. 486
17.2.11 CELLECTA, INC. 487
17.2.12 APPLIED STEMCELL 488
18 APPENDIX 489
18.1 DISCUSSION GUIDE 489
18.2 KNOWLEDGESTORE: MARKETSANDMARKETS’ SUBSCRIPTION PORTAL 494
18.3 CUSTOMIZATION OPTIONS 496
18.4 RELATED REPORTS 496
18.5 AUTHOR DETAILS 497
❖ 世界の遺伝子編集市場に関するよくある質問(FAQ) ❖
・遺伝子編集の世界市場規模は?
→MarketsandMarkets社は2024年の遺伝子編集の世界市場規模を46.6億米ドルと推定しています。
・遺伝子編集の世界市場予測は?
→MarketsandMarkets社は2029年の遺伝子編集の世界市場規模を75.9億米ドルと予測しています。
・遺伝子編集市場の成長率は?
→MarketsandMarkets社は遺伝子編集の世界市場が2024年~2029年に年平均10.2%成長すると予測しています。
・世界の遺伝子編集市場における主要企業は?
→MarketsandMarkets社は「Thermo Fisher Scientific Inc. (US)、Merck KGaA (Germany)、GenScript (US)、Agilent Technologies、Inc. (US)、Revvity (US)、Lonza (US)、Tecan Trading AG (Switzerland)、Sangamo therapeutics (US). Precision BioSciences (US)、Cellectis S.A. (France)、Regeneron Pharmaceuticals Inc. (US)、AMSBIO (UK)、Creative Biogene (US)、Synthego (US)、Takara Bio Inc. (Japan)、Bio-Techne (US)、Caribou Biosciences、Inc. (US) Bioneer corporation (South Korea)、and REPROCELL Inc. (Japan).など ...」をグローバル遺伝子編集市場の主要企業として認識しています。
※上記FAQの市場規模、市場予測、成長率、主要企業に関する情報は本レポートの概要を作成した時点での情報であり、納品レポートの情報と少し異なる場合があります。