1 はじめに 26
1.1 調査目的 26
1.2 市場の定義 26
1.3 調査範囲 27
1.3.1 対象市場と地域範囲 27
1.3.2 対象範囲と除外項目 28
1.3.3 考慮した年数 28
1.4 考慮した通貨 28
1.5 単位の考慮 28
1.6 利害関係者 29
1.7 変更点のまとめ 29
2 調査方法 30
2.1 調査データ 30
2.1.1 二次データ 31
2.1.1.1 主要な二次情報源のリスト 31
2.1.1.2 二次資料からの主要データ 31
2.1.2 一次データ 32
2.1.2.1 一次資料からの主要データ 32
2.1.2.2 主要な一次インタビュー参加者のリスト 33
2.1.2.3 主要な業界インサイト 33
2.1.2.4 専門家へのインタビューの内訳 33
2.2 市場規模の推定 34
2.2.1 ボトムアップアプローチ 34
2.2.2 トップダウンアプローチ 35
2.3 成長予測 36
2.4 データの三角測量 37
2.5 要因分析 38
2.6 リサーチの前提 38
2.7 調査の限界とリスク分析 39
3 エグゼクティブサマリー 40
4 プレミアムインサイト 44
4.1 医療用エラストマー市場におけるプレーヤーにとっての魅力的な機会 44
4.2 医療用エラストマー市場:タイプ別、2024年対2029年(キロトン) 44
4.3 医療用エラストマー市場:用途別(2024年対2029年)(キロトン) 45
4.4 医療用エラストマー市場:最終用途産業別、2024年対2029年(キロトン) 45
4.5 医療用エラストマー市場:主要国別 46
5 市場概要 47
5.1 はじめに 47
5.2 市場ダイナミクス 47
5.2.1 推進要因 48
5.2.1.1 医療機器の需要拡大 48
5.2.1.2 医療における技術の進歩 48
5.2.1.3 慢性疾患の蔓延 49
5.2.2 阻害要因 49
5.2.2.1 高い製造コスト 49
5.2.3 機会 50
5.2.3.1 新興国における医療投資の増加 50
5.2.3.2 低侵襲デバイスでの使用の増加 50
5.2.4 課題 50
5.2.4.1 厳しい規制要件 50
5.3 ポーターの5つの力分析 51
5.3.1 新規参入による脅威 52
5.3.2 代替品の脅威 52
5.3.3 供給者の交渉力 52
5.3.4 買い手の交渉力 53
5.3.5 競合の激しさ 53
5.4 主要ステークホルダーと購買基準 54
5.4.1 購買プロセスにおける主要ステークホルダー 54
5.4.2 購買基準 55
5.5 マクロ経済の見通し 56
5.5.1 GDPの動向と予測 56
5.6 AI/ジェナイの影響 59
5.7 バリューチェーン分析 60
5.8 エコシステム分析 61
5.9 ケーススタディ分析 62
5.9.1 非ラテックス医療部品への移行を支援したケント・エラストマー 62
5.9.2 クーパー大学病院がデンテック・セーフティコンフォート・エアンクス MD でポリフェニレンエーテルの回復力を強化 63
5.9.3 整形外科用途でシリコーンに代わる高性能材料としてのテクノフロン FK 63
5.10 規制の状況 64
5.10.1 環境規制 64
5.10.2 北米 64
5.10.3 アジア太平洋地域 65
5.10.4 欧州 66
5.10.5 規制機関、政府機関、その他の組織 67
5.11 技術分析 68
5.11.1 主要技術 68
5.11.1.1 押出技術 68
5.11.1.2 圧縮成形 68
5.11.2 補足技術 68
5.11.2.1 共押出技術 68
5.11.3 隣接技術 69
5.11.3.1 積層造形技術 69
5.12 顧客ビジネスに影響を与えるトレンド/破壊 69
5.13 貿易分析 70
5.13.1 輸入シナリオ(HSコード 391000) 70
5.13.2 輸出シナリオ(HS コード 391000) 71
5.13.3 輸入シナリオ(HSコード400270) 72
5.13.4 輸出シナリオ(HSコード400270) 73
5.14 主要会議・イベント(2024-2025年) 74
5.15 価格分析 75
5.15.1 医療用エラストマーの地域別平均販売価格動向 75
5.15.2 医療用エラストマーの平均販売価格動向(用途別) 76
用途別 76
5.15.3 医療用エラストマーの平均販売価格動向(用途別) 76
最終用途産業別 76
5.16 投資と資金調達のシナリオ 77
5.17 特許分析 77
5.17.1 導入 77
5.17.2 特許の法的地位 79
5.17.3 管轄区域分析 80
5.18 潜在顧客のリスト 80
6 医療用エラストマー市場:技術別 83
6.1 導入 84
6.2 押出成形 84
6.3 射出成形 84
6.4 圧縮成形 84
6.5 その他の技術 85
7 医療用エラストマー市場:最終用途産業別 86
7.1 はじめに 87
7.2 病院・診療所 89
7.2.1 慢性疾患の蔓延が市場を牽引 89
7.3 医薬品 89
7.3.1 包装と薬物送達システムにおけるエラストマーの重要な役割が普及を促進 89
7.4 医療機器製造 90
7.4.1 先端医療機器への需要の高まりが市場成長を後押し 90
7.5 その他の最終用途産業 90
8 医療用エラストマー市場:用途別 91
8.1 はじめに 92
8.2 医療用チューブ 96
8.2.1 先進的医療用チュービング・ソリューションにおける高い消費が市場を牽引 96
8.3 カテーテル 97
8.3.1 カテーテル製造における重要な役割が成長を促進 97
8.4 グローブ 97
8.4.1 個人用保護具のニーズの高まりが需要を押し上げる 97
8.5 注射器 97
8.5.1 プレフィルドシリンジ 98
8.5.1.1 投与ミスのリスク低減:採用の主要因 98
8.5.2 非充填シリンジ 98
8.5.2.1 慢性疾患の罹患率の増加が採用を促進 98
8.5.3 バイアル 98
8.5.3.1 安全で効果的な薬物送達システムに対するニーズの増加が市場を牽引 98
8.6 医療・輸液バッグ 99
8.6.1 院内感染の増加が市場成長を支える 99
8.7 インプラント 99
8.7.1 加齢に伴う健康状態の蔓延が市場を牽引 99
8.8 その他の用途 100
9 医療用エラストマー市場:タイプ別 101
9.1 はじめに 102
9.2 熱硬化性エラストマー 104
9.2.1 シリコーン 104
9.2.1.1 医療・医薬用途におけるラテックス製品の効率的代替材料 104
9.2.1.2 高温加硫 105
9.2.1.3 液状シリコーンゴム 105
9.2.1.4 常温加硫 105
9.2.2 エチレンプロピレンジエンモノマー 106
9.2.2.1 様々な医療用途に適し、市場成長を支える主な要因 106
9.2.3 その他の熱硬化性エラストマー 106
9.3 熱可塑性エラストマー 106
9.3.1 熱可塑性ポリウレタン 107
9.3.1.1 押出成形技術による複雑な設計の製造の効率化 107
9.3.2 スチレンブロック共重合体 107
9.3.2.1 SBCの生体安定性が医療用途での有用性を高める-市場成長 の主要因 107
9.3.3 その他の熱可塑性エラストマー 107
10 医療用エラストマー市場:地域別 108
10.1 はじめに 109
10.2 北米 111
10.2.1 米国 121
10.2.1.1 医療支出の増加が市場を牽引 121
10.2.2 カナダ 124
10.2.2.1 拡大する医療制度が市場成長を支える 124
10.2.3 メキシコ 128
10.2.3.1 政府のイニシアティブと医療セクターの成長が市場を牽引 128
10.3 アジア太平洋地域 132
10.3.1 中国 142
10.3.1.1 高齢化の進展が市場を牽引 142
10.3.2 日本 145
10.3.2.1 感染対策と患者の安全性への関心の高まりが市場を促進 145
10.3.3 インド 149
10.3.3.1 医療分野への投資の増加が市場を牽引 149
10.3.4 韓国 153
10.3.4.1 高品質医療機器への需要増加が市場を牽引 153
10.3.5 インドネシア 157
10.3.5.1 慢性疾患の増加が市場を牽引 157
10.3.6 その他のアジア太平洋地域 161
10.4 欧州 165
10.4.1 ドイツ 175
10.4.1.1 研究開発投資の増加が市場を牽引 175
10.4.2 イギリス 178
10.4.2.1 高齢者人口の増加が市場成長を支える 178
10.4.3 フランス 182
10.4.3.1 大手医療機器メーカーの存在が市場を促進 182
10.4.4 イタリア 186
10.4.4.1 公立病院からの医療機器需要が増加 186
10.4.5 スペイン 190
10.4.5.1 医療インフラへの投資の増加が市場を牽引 190
10.4.6 その他のヨーロッパ 194
10.5 中東・アフリカ 198
10.5.1 GCC諸国 207
10.5.1.1 サウジアラビア 207
10.5.1.1.1 先端医療技術のニーズが市場を牽引 207
10.5.1.2 その他のGCC諸国 211
10.5.2 南アフリカ 215
10.5.2.1 伝染病の発生が市場を牽引 215
10.5.3 その他の中東・アフリカ 219
10.6 南米 223
10.6.1 ブラジル 232
10.6.1.1 南米最大の医療用エラストマー市場 232
10.6.2 アルゼンチン 235
10.6.2.1 医療機器需要の増加が市場を牽引 235
10.6.3 南米のその他地域 239
11 競争環境 244
11.1 概要 244
11.2 主要プレーヤーの戦略/勝利への権利(2019~2024年) 245
11.3 収益分析、2021-2023年 246
11.4 市場シェア分析、2023年 247
11.4.1 北米:用途別市場シェア分析(2023年) 248
11.4.2 欧州: 市場シェア分析、用途別、2023年 249
11.4.3 アジア太平洋地域:用途別市場シェア分析、2023年 250
11.4.4 DOW 250
11.4.5 エクソンモービル・コーポレーション 251
11.4.6 セラニーズコーポレーション 251
11.4.7 BASF 251
11.4.8 ワッカー・ケミー 251
11.5 企業評価と財務指標 252
11.6 ブランド/製品の比較 253
11.6.1 医療用エラストマー市場:ブランド/製品比較分析 253
11.6.2 エラストラン 253
11.6.3 エンゲージ 8480K ヘルスプラス 253
11.6.4 ハイトレル 254
11.6.5 エクデル 254
11.7 企業評価マトリックス:主要企業(2023年) 254
11.7.1 スター企業 254
11.7.2 新興リーダー 254
11.7.3 浸透型プレーヤー 254
11.7.4 参加企業 254
11.7.5 企業フットプリント:主要プレーヤー、2023年 256
11.7.5.1 全体的な企業フットプリント 256
11.7.5.2 地域別フットプリント 257
11.7.5.3 技術のフットプリント 258
11.7.5.4 タイプ別フットプリント 259
11.7.5.5 アプリケーションフットプリント 260
11.7.5.6 産業フットプリント 261
11.8 企業評価マトリクス:新興企業/SM(2023年) 262
11.8.1 進歩的企業 262
11.8.2 対応力のある企業 262
11.8.3 ダイナミックな企業 262
11.8.4 スタートアップ・ブロック 262
11.8.5 競争ベンチマーキング:新興企業/SM(2023年) 264
11.8.5.1 新興企業/中小企業の詳細リスト 264
11.8.5.2 新興企業/中小企業の競合ベンチマーキング 265
11.9 競争シナリオ 267
11.9.1 製品上市 267
11.9.2 取引 268
11.9.3 拡張 269
11.9.4 その他の開発 270
12 企業プロフィール 271
12.1 主要企業 271
BASF (Germany)
Dow (US)
Celanese Corporation (US)
Eastman Chemical Company (US)
Syensqo (Belgium)
Mitsubishi Chemical Group Corporation (Japan)
Kuraray Co.Ltd. (Japan)
ExxonMobil (US)
Momentive Performance Materials (US)
Envalior (Germany) and Zeon Corporation (Japan)
13 付録 326
13.1 ディスカッションガイド 326
13.2 Knowledgestore: Marketsandmarketsの購読ポータル 329
13.3 カスタマイズオプション 331
13.4 関連レポート 331
13.5 著者の詳細 332

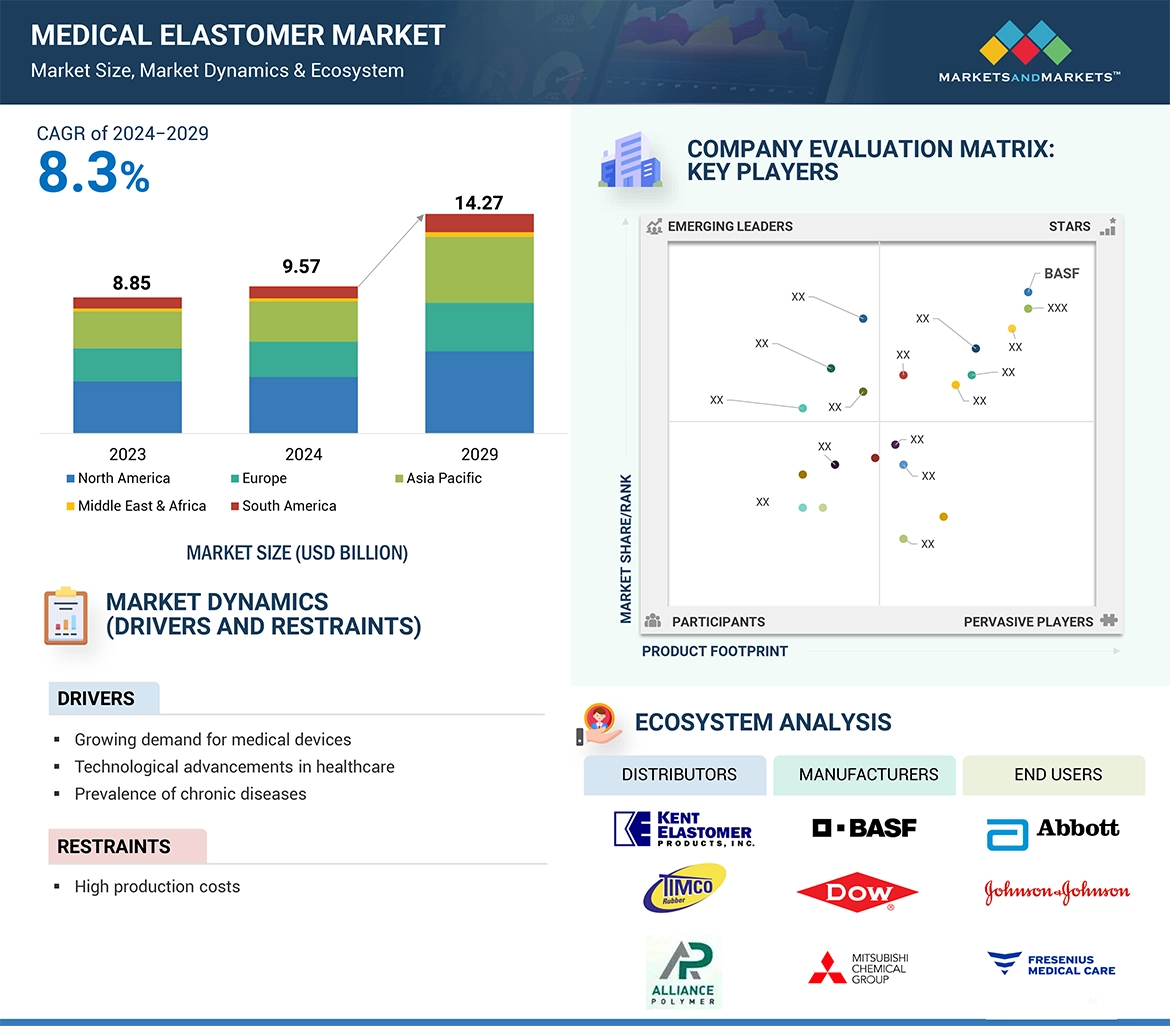
Growing demand for medical devices is fueling the demand for medical elastomers worldwide. This is a result of the increased use in disposable medical equipment, such as syringes, valves, tubing, and critical implants; artificial heart valves and artificial joints. These materials are versatile, offering durability, biocompatibility, and flexibility, making them suitable in a wide range of medical applications, such as medical tubes, bags, and gloves. The growing healthcare costs in developing economies coupled with technology advancements will further fuel the increasing demand for these critical commodities, rendering them even more indispensable to meet growing healthcare needs across the world. Additionally, the expanding population in emerging economies along with the rising prevalence of chronic diseases is creating a demand for medical elastomers.
“Based on application, implants are the fastest growing application in medical elastomer market during the forecast period, in terms of value.”
Implants are the fastest growing application of medical elastomers due to increasing demand for biocompatible, durable and flexible materials in medical procedures. The rising prevalence of chronic diseases and aging populations are driving the need of durable implants in medical elastomers, as it provides superior orthopedic, dental, and soft tissue implant performance. Flexibility and comfort with least complications further make them appealing. Moreover, the ability of elastomers to be molded into complex shapes and the excellent biocompatibility enhances the future applications of customized implants, thereby further fueling the demand for medical elastomers.
“Based on end-use industry, hospitals & clinics account the largest share in medical elastomer market during the forecast period, in terms of value.”
Hospitals and clinics account for the largest share in the medical elastomer market due to their heavy dependence on medical devices and equipment that utilize elastomer materials. These institutions need a wide range of products based on medical elastomers such as catheters, medical tubing, seals, gaskets, surgical gloves, and implants, in order to ensure appropriate patient care and treatment. However, the growing need for healthcare services due to growing ageing population and rising prevalence of chronic diseases such as diabetes, cardiovascular diseases, and respiratory disorders further boosts the demand for these medical devices. The properties of medical elastomers such as biocompatibility and excellent flexibility & durability along with chemical resistance makes them highly functional for various applications in hospital and clinic applications. These factors, along with the stringent regulatory requirements from healthcare authorities like FDA, ensure that hospitals and clinics are adopting high-quality elastomer-based products. All these factors combined strong healthcare needs, adherence to regulatory requirements, and the drive for advanced, reliable medical devices ensure make hospitals & clinics account largest market share by industry in the medical elastomer market.
“Based on region, Asia Pacific accounts the second largest market for medical elastomer, in terms of value.”
Asia Pacific accounts the second largest market for medical elastomers because of various factors such as economic development, rapid population growth, a good healthcare sector, and increasing demand for medical devices. The rise in investments for healthcare infrastructure in emerging economies like China and India is driving the demand for adoption high-quality medical elastomers into healthcare equipments such as medical tubing, implants, and diagnostic equipment. Large and aging population in the region have added up to this demand as prevalence of chronic diseases is rising among this population. Asia Pacific is, therefore, a growth product concerning medical facility organizations dedicated to better healthcare access, positioning it as a major player in the global medical elastomer market.
In the process of determining and verifying the market size for several segments and subsegments identified through secondary research, extensive primary interviews were conducted. A breakdown of the profiles of the primary interviewees are as follows:
• By Company Type: Tier 1 - 40%, Tier 2 - 30%, and Tier 3 - 30%
• By Designation: Directors- 35%, Managers - 25%, and Others - 40%
• By Region: North America - 22%, Europe - 22%, Asia Pacific - 45%, RoW – 11%
The key players in this market are BASF (Germany), Dow (US), Celanese Corporation (US), Eastman Chemical Company (US), Syensqo (Belgium), Mitsubishi Chemical Group Corporation (Japan), Kuraray Co., Ltd. (Japan), ExxonMobil (US), Momentive Performance Materials (US), Envalior (Germany) and Zeon Corporation (Japan).
Research Coverage
This report segments the medical elastomer market based on type, technology, application, end-use industry, and region, and provides estimations for the overall value of the market across various regions. A detailed analysis of key industry players has been conducted to provide insights into their business overviews, products and services, key strategies, new product launches, expansions, and mergers and acquisitions associated with the medical elastomer market.
Key benefits of buying this report
This research report focuses on various levels of analysis, including industry analysis (industry trends), market ranking analysis of top players, and company profiles, which together provide an overall view of the competitive landscape, emerging and high-growth segments of the medical elastomer market, high-growth regions, and market drivers, restraints, opportunities, and challenges.
The report provides insights on the following pointers:
• Analysis of key drivers (Growing demand for medical devices, Technological advancements in healthcare and Prevalence of chronic diseases), restraints (High production costs), opportunities (Growing healthcare investment in emerging economies) and challenges (Stringent regulatory requirements).
• Market Penetration: Comprehensive information on the medical elastomer market offered by top players in the global medical elastomer market.
• Product Development/Innovation: Detailed insights on upcoming technologies, research & development activities, and new product launches in the medical elastomer market.
• Market Development: Comprehensive information about lucrative emerging markets — the report analyzes the markets for medical elastomer market across regions.
• Market Diversification: Exhaustive information about new products, untapped regions, and recent developments in the global medical elastomer market
• Competitive Assessment: In-depth assessment of market shares, strategies, products, and manufacturing capabilities of leading players in the medical elastomer market
1 INTRODUCTION 26
1.1 STUDY OBJECTIVES 26
1.2 MARKET DEFINITION 26
1.3 STUDY SCOPE 27
1.3.1 MARKETS COVERED AND REGIONAL SCOPE 27
1.3.2 INCLUSIONS AND EXCLUSIONS 28
1.3.3 YEARS CONSIDERED 28
1.4 CURRENCY CONSIDERED 28
1.5 UNITS CONSIDERED 28
1.6 STAKEHOLDERS 29
1.7 SUMMARY OF CHANGES 29
2 RESEARCH METHODOLOGY 30
2.1 RESEARCH DATA 30
2.1.1 SECONDARY DATA 31
2.1.1.1 List of key secondary sources 31
2.1.1.2 Key data from secondary sources 31
2.1.2 PRIMARY DATA 32
2.1.2.1 Key data from primary sources 32
2.1.2.2 List of key primary interview participants 33
2.1.2.3 Key industry insights 33
2.1.2.4 Breakdown of interviews with experts 33
2.2 MARKET SIZE ESTIMATION 34
2.2.1 BOTTOM-UP APPROACH 34
2.2.2 TOP-DOWN APPROACH 35
2.3 GROWTH FORECAST 36
2.4 DATA TRIANGULATION 37
2.5 FACTOR ANALYSIS 38
2.6 RESEARCH ASSUMPTIONS 38
2.7 RESEARCH LIMITATIONS AND RISK ANALYSIS 39
3 EXECUTIVE SUMMARY 40
4 PREMIUM INSIGHTS 44
4.1 ATTRACTIVE OPPORTUNITIES FOR PLAYERS IN MEDICAL ELASTOMERS MARKET 44
4.2 MEDICAL ELASTOMERS MARKET, BY TYPE, 2024 VS. 2029 (KILOTON) 44
4.3 MEDICAL ELASTOMERS MARKET, BY APPLICATION, 2024 VS. 2029 (KILOTON) 45
4.4 MEDICAL ELASTOMERS MARKET, BY END-USE INDUSTRY, 2024 VS. 2029 (KILOTON) 45
4.5 MEDICAL ELASTOMERS MARKET, BY KEY COUNTRY 46
5 MARKET OVERVIEW 47
5.1 INTRODUCTION 47
5.2 MARKET DYNAMICS 47
5.2.1 DRIVERS 48
5.2.1.1 Growing demand for medical devices 48
5.2.1.2 Technological advancements in healthcare 48
5.2.1.3 Prevalence of chronic diseases 49
5.2.2 RESTRAINTS 49
5.2.2.1 High production costs 49
5.2.3 OPPORTUNITIES 50
5.2.3.1 Growing healthcare investments in emerging economies 50
5.2.3.2 Increasing use in minimally invasive devices 50
5.2.4 CHALLENGES 50
5.2.4.1 Stringent regulatory requirements 50
5.3 PORTER’S FIVE FORCES ANALYSIS 51
5.3.1 THREAT FROM NEW ENTRANTS 52
5.3.2 THREAT OF SUBSTITUTES 52
5.3.3 BARGAINING POWER OF SUPPLIERS 52
5.3.4 BARGAINING POWER OF BUYERS 53
5.3.5 INTENSITY OF COMPETITIVE RIVALRY 53
5.4 KEY STAKEHOLDERS AND BUYING CRITERIA 54
5.4.1 KEY STAKEHOLDERS IN BUYING PROCESS 54
5.4.2 BUYING CRITERIA 55
5.5 MACROECONOMIC OUTLOOK 56
5.5.1 GDP TRENDS AND FORECASTS 56
5.6 IMPACT OF AI/GENAI 59
5.7 VALUE CHAIN ANALYSIS 60
5.8 ECOSYSTEM ANALYSIS 61
5.9 CASE STUDY ANALYSIS 62
5.9.1 KENT ELASTOMERS HELPED IN TRANSITIONING TO NON-LATEX MEDICAL COMPONENTS 62
5.9.2 COOPER UNIVERSITY HOSPITAL BOOSTS POLYPHENYLENE ETHER RESILIENCE WITH DENTEC SAFETY COMFORT-AIRNXMD 63
5.9.3 TECNOFLON FKM AS HIGH-PERFORMANCE ALTERNATIVE TO SILICONE IN ORTHOPEDIC APPLICATIONS 63
5.10 REGULATORY LANDSCAPE 64
5.10.1 ENVIRONMENTAL REGULATIONS 64
5.10.2 NORTH AMERICA 64
5.10.3 ASIA PACIFIC 65
5.10.4 EUROPE 66
5.10.5 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS 67
5.11 TECHNOLOGY ANALYSIS 68
5.11.1 KEY TECHNOLOGIES 68
5.11.1.1 Extrusion technology 68
5.11.1.2 Compression molding 68
5.11.2 COMPLEMENTARY TECHNOLOGIES 68
5.11.2.1 Co-extrusion technology 68
5.11.3 ADJACENT TECHNOLOGIES 69
5.11.3.1 Additive manufacturing technology 69
5.12 TRENDS/DISRUPTIONS IMPACTING CUSTOMER BUSINESS 69
5.13 TRADE ANALYSIS 70
5.13.1 IMPORT SCENARIO (HS CODE 391000) 70
5.13.2 EXPORT SCENARIO (HS CODE 391000) 71
5.13.3 IMPORT SCENARIO (HS CODE 400270) 72
5.13.4 EXPORT SCENARIO (HS CODE 400270) 73
5.14 KEY CONFERENCES AND EVENTS, 2024–2025 74
5.15 PRICING ANALYSIS 75
5.15.1 AVERAGE SELLING PRICE TREND OF MEDICAL ELASTOMERS, BY REGION 75
5.15.2 AVERAGE SELLING PRICE TREND OF MEDICAL ELASTOMERS,
BY APPLICATION 76
5.15.3 AVERAGE SELLING PRICE TREND OF MEDICAL ELASTOMERS,
BY END-USE INDUSTRY 76
5.16 INVESTMENT AND FUNDING SCENARIO 77
5.17 PATENT ANALYSIS 77
5.17.1 INTRODUCTION 77
5.17.2 LEGAL STATUS OF PATENTS 79
5.17.3 JURISDICTION ANALYSIS 80
5.18 LIST OF POTENTIAL CUSTOMERS 80
6 MEDICAL ELASTOMERS MARKET, BY TECHNOLOGY 83
6.1 INTRODUCTION 84
6.2 EXTRUSION 84
6.3 INJECTION MOLDING 84
6.4 COMPRESSION MOLDING 84
6.5 OTHER TECHNOLOGIES 85
7 MEDICAL ELASTOMERS MARKET, BY END-USE INDUSTRY 86
7.1 INTRODUCTION 87
7.2 HOSPITALS & CLINICS 89
7.2.1 RISING PREVALENCE OF CHRONIC DISEASES TO DRIVE MARKET 89
7.3 PHARMACEUTICAL 89
7.3.1 CRUCIAL ROLE OF ELASTOMERS IN PACKAGING AND DRUG DELIVERY SYSTEMS TO PROPEL ADOPTION 89
7.4 MEDICAL DEVICE MANUFACTURING 90
7.4.1 RISING DEMAND FOR ADVANCED MEDICAL DEVICES TO SUPPORT MARKET GROWTH 90
7.5 OTHER END-USE INDUSTRIES 90
8 MEDICAL ELASTOMERS MARKET, BY APPLICATION 91
8.1 INTRODUCTION 92
8.2 MEDICAL TUBING 96
8.2.1 HIGH CONSUMPTION IN ADVANCING MEDICAL TUBING SOLUTIONS TO DRIVE MARKET 96
8.3 CATHETERS 97
8.3.1 VITAL ROLE IN PRODUCTION OF CATHETERS TO PROPEL GROWTH 97
8.4 GLOVES 97
8.4.1 INCREASING NEED FOR PERSONAL PROTECTIVE EQUIPMENT TO INCREASE DEMAND 97
8.5 SYRINGES 97
8.5.1 PRE-FILLED SYRINGES 98
8.5.1.1 Reduce risk of dosing errors—key factor driving adoption 98
8.5.2 NON-PRE-FILLED SYRINGES 98
8.5.2.1 Increasing incidence of chronic diseases to propel adoption 98
8.5.3 VIALS 98
8.5.3.1 Increasing need for safe and effective drug delivery systems to drive market 98
8.6 MEDICAL & INFUSION BAGS 99
8.6.1 RISE IN HOSPITAL-ACQUIRED INFECTIONS TO SUPPORT MARKET GROWTH 99
8.7 IMPLANTS 99
8.7.1 INCREASING PREVALENCE OF AGE-RELATED HEALTH CONDITIONS TO DRIVE MARKET 99
8.8 OTHER APPLICATIONS 100
9 MEDICAL ELASTOMERS MARKET, BY TYPE 101
9.1 INTRODUCTION 102
9.2 THERMOSET ELASTOMERS 104
9.2.1 SILICONE 104
9.2.1.1 Efficient alternative to latex products in medical and pharmaceutical applications 104
9.2.1.2 High temperature vulcanized 105
9.2.1.3 Liquid silicone rubber 105
9.2.1.4 Room temperature vulcanized 105
9.2.2 ETHYLENE PROPYLENE DIENE MONOMER 106
9.2.2.1 Suitable for various medical applications—key factor supporting market growth 106
9.2.3 OTHER THERMOSET ELASTOMERS 106
9.3 THERMOPLASTIC ELASTOMERS 106
9.3.1 THERMOPLASTIC POLYURETHANE 107
9.3.1.1 Provide efficiency in manufacturing complex designs using extrusion and molding techniques 107
9.3.2 STYRENE BLOCK COPOLYMERS 107
9.3.2.1 Biostability of SBC increases utility in medical applications—key factor driving market growth 107
9.3.3 OTHER THERMOPLASTIC ELASTOMERS 107
10 MEDICAL ELASTOMERS MARKET, BY REGION 108
10.1 INTRODUCTION 109
10.2 NORTH AMERICA 111
10.2.1 US 121
10.2.1.1 Rising healthcare spending to drive market 121
10.2.2 CANADA 124
10.2.2.1 Expanding healthcare system to support market growth 124
10.2.3 MEXICO 128
10.2.3.1 Government initiatives and growth of healthcare sector to drive market 128
10.3 ASIA PACIFIC 132
10.3.1 CHINA 142
10.3.1.1 Growing aging population to drive market 142
10.3.2 JAPAN 145
10.3.2.1 Increased focus on infection control and patient safety to propel market 145
10.3.3 INDIA 149
10.3.3.1 Increasing investments in healthcare sector to drive market 149
10.3.4 SOUTH KOREA 153
10.3.4.1 Rising demand for high quality medical devices to drive market 153
10.3.5 INDONESIA 157
10.3.5.1 Rising incidence of chronic diseases to drive market 157
10.3.6 REST OF ASIA PACIFIC 161
10.4 EUROPE 165
10.4.1 GERMANY 175
10.4.1.1 Increased investments in R&D to drive market 175
10.4.2 UK 178
10.4.2.1 Rising geriatric population to support market growth 178
10.4.3 FRANCE 182
10.4.3.1 Presence of significant medical device manufacturers to propel market 182
10.4.4 ITALY 186
10.4.4.1 Rising demand for medical devices from public hospitals to increase demand 186
10.4.5 SPAIN 190
10.4.5.1 Increasing investments in healthcare infrastructure to propel market 190
10.4.6 REST OF EUROPE 194
10.5 MIDDLE EAST & AFRICA 198
10.5.1 GCC COUNTRIES 207
10.5.1.1 Saudi Arabia 207
10.5.1.1.1 Need for advanced medical technologies to drive market 207
10.5.1.2 Rest of GCC countries 211
10.5.2 SOUTH AFRICA 215
10.5.2.1 Outbreak of contagious diseases to drive market 215
10.5.3 REST OF MIDDLE EAST & AFRICA 219
10.6 SOUTH AMERICA 223
10.6.1 BRAZIL 232
10.6.1.1 Largest market for medical elastomers in South America 232
10.6.2 ARGENTINA 235
10.6.2.1 Growing demand for medical devices to drive market 235
10.6.3 REST OF SOUTH AMERICA 239
11 COMPETITIVE LANDSCAPE 244
11.1 OVERVIEW 244
11.2 KEY PLAYER STRATEGIES/RIGHT TO WIN, 2019–2024 245
11.3 REVENUE ANALYSIS, 2021–2023 246
11.4 MARKET SHARE ANALYSIS, 2023 247
11.4.1 NORTH AMERICA: MARKET SHARE ANALYSIS, BY APPLICATION, 2023 248
11.4.2 EUROPE: MARKET SHARE ANALYSIS, BY APPLICATION, 2023 249
11.4.3 ASIA PACIFIC: MARKET SHARE ANALYSIS, BY APPLICATION, 2023 250
11.4.4 DOW 250
11.4.5 EXXON MOBIL CORPORATION 251
11.4.6 CELANESE CORPORATION 251
11.4.7 BASF 251
11.4.8 WACKER CHEMIE AG 251
11.5 COMPANY VALUATION AND FINANCIAL METRICS 252
11.6 BRAND/PRODUCT COMPARISON 253
11.6.1 MEDICAL ELASTOMERS MARKET: BRAND/PRODUCT COMPARISON ANALYSIS 253
11.6.2 ELASTOLLAN 253
11.6.3 ENGAGE 8480K HEALTH+ 253
11.6.4 HYTREL 254
11.6.5 ECDEL 254
11.7 COMPANY EVALUATION MATRIX: KEY PLAYERS, 2023 254
11.7.1 STARS 254
11.7.2 EMERGING LEADERS 254
11.7.3 PERVASIVE PLAYERS 254
11.7.4 PARTICIPANTS 254
11.7.5 COMPANY FOOTPRINT: KEY PLAYERS, 2023 256
11.7.5.1 Overall company footprint 256
11.7.5.2 Region footprint 257
11.7.5.3 Technology footprint 258
11.7.5.4 Type Footprint 259
11.7.5.5 Application footprint 260
11.7.5.6 Industry footprint 261
11.8 COMPANY EVALUATION MATRIX: STARTUPS/SMES, 2023 262
11.8.1 PROGRESSIVE COMPANIES 262
11.8.2 RESPONSIVE COMPANIES 262
11.8.3 DYNAMIC COMPANIES 262
11.8.4 STARTING BLOCKS 262
11.8.5 COMPETITIVE BENCHMARKING: STARTUPS/SMES, 2023 264
11.8.5.1 Detailed list of startups/SMEs 264
11.8.5.2 Competitive benchmarking of startups/SMEs 265
11.9 COMPETITIVE SCENARIO 267
11.9.1 PRODUCT LAUNCHES 267
11.9.2 DEALS 268
11.9.3 EXPANSIONS 269
11.9.4 OTHER DEVELOPMENTS 270
12 COMPANY PROFILES 271
12.1 KEY PLAYERS 271
12.1.1 DOW 271
12.1.1.1 Business overview 271
12.1.1.2 Products offered 272
12.1.1.3 Recent developments 273
12.1.1.3.1 Other developments 273
12.1.1.4 MnM view 273
12.1.1.4.1 Key strengths 273
12.1.1.4.2 Strategic choices 273
12.1.1.4.3 Weaknesses and competitive threats 274
12.1.2 EXXON MOBIL CORPORATION 274
12.1.2.1 Business overview 274
12.1.2.2 Products offered 276
12.1.2.3 MnM view 276
12.1.2.3.1 Key strengths 276
12.1.2.3.2 Strategic choices 276
12.1.2.3.3 Weaknesses and competitive threats 276
12.1.3 CELANESE CORPORATION 277
12.1.3.1 Business overview 277
12.1.3.2 Products offered 278
12.1.3.3 Recent developments 279
12.1.3.3.1 Deals 279
12.1.3.4 MnM view 280
12.1.3.4.1 Key strengths 280
12.1.3.4.2 Strategic choices 280
12.1.3.4.3 Weaknesses and competitive threats 280
12.1.4 BASF 281
12.1.4.1 Business overview 281
12.1.4.2 Products offered 282
12.1.4.3 Recent developments 283
12.1.4.3.1 Expansions 283
12.1.4.4 MnM view 283
12.1.4.4.1 Key strengths 283
12.1.4.4.2 Strategic choices 284
12.1.4.4.3 Weaknesses and competitive threats 284
12.1.5 WACKER CHEMIE AG 285
12.1.5.1 Business overview 285
12.1.5.2 Products offered 286
12.1.5.3 Recent developments 287
12.1.5.3.1 Product launches 287
12.1.5.3.2 Expansions 288
12.1.5.4 MnM view 289
12.1.5.4.1 Key strengths 289
12.1.5.4.2 Strategic choices 289
12.1.5.4.3 Weaknesses and competitive threats 289
12.1.6 EASTMAN CHEMICAL COMPANY 290
12.1.6.1 Business overview 290
12.1.6.2 Products offered 291
12.1.6.3 MnM view 291
12.1.6.3.1 Key strengths 291
12.1.6.3.2 Strategic choices 292
12.1.6.3.3 Weaknesses and competitive threats 292
12.1.7 SYENSQO 293
12.1.7.1 Business overview 293
12.1.7.2 Products offered 294
12.1.7.3 Recent developments 295
12.1.7.3.1 Other developments 295
12.1.7.4 MnM view 295
12.1.7.4.1 Key strengths 295
12.1.7.4.2 Strategic choices 295
12.1.7.4.3 Weaknesses and competitive threats 296
12.1.8 KURARAY CO., LTD 297
12.1.8.1 Business overview 297
12.1.8.2 Products offered 298
12.1.8.3 MnM view 299
12.1.8.3.1 Key strengths 299
12.1.8.3.2 Strategic choices 299
12.1.8.3.3 Weaknesses and competitive threats 299
12.1.9 MITSUBISHI CHEMICAL GROUP CORPORATION 300
12.1.9.1 Business overview 300
12.1.9.2 Products offered 301
12.1.9.3 Recent developments 302
12.1.9.3.1 Other developments 302
12.1.9.3.2 Deals 303
12.1.9.4 MnM view 303
12.1.10 MOMENTIVE PERFORMANCE MATERIALS 304
12.1.10.1 Business overview 304
12.1.10.2 Products offered 304
12.1.10.3 MnM view 305
12.1.11 ENVALIOR 306
12.1.11.1 Business overview 306
12.1.11.2 Products offered 306
12.1.12 ZEON CORPORATION 308
12.1.12.1 Business overview 308
12.1.12.2 Products offered 309
12.1.13 THE LUBRIZOL CORPORATION 310
12.1.13.1 Business overview 310
12.1.13.2 Products offered 310
12.1.13.3 MnM view 311
12.2 OTHER PLAYERS 312
12.2.1 KRATON CORPORATION 312
12.2.2 FOSTER CORPORATION 313
12.2.3 BIOMERICS 314
12.2.4 RTP COMPANY 315
12.2.5 ROMAR ENGINEERING 316
12.2.6 THE RUBBER GROUP 317
12.2.7 KENT ELASTOMER PRODUCTS 318
12.2.8 RAUMEDIC AG 319
12.2.9 THE HYGENIC COMPANY, LLC 320
12.2.10 HEXAPOL AB 321
12.2.11 TEKNI-PLEX, INC 322
12.2.12 TRINSEO 323
12.2.13 TRELLEBORG AB 324
12.2.14 SAINT-GOBAIN 325
13 APPENDIX 326
13.1 DISCUSSION GUIDE 326
13.2 KNOWLEDGESTORE: MARKETSANDMARKETS’ SUBSCRIPTION PORTAL 329
13.3 CUSTOMIZATION OPTIONS 331
13.4 RELATED REPORTS 331
13.5 AUTHOR DETAILS 332
❖ 世界の医療用エラストマー市場に関するよくある質問(FAQ) ❖
・医療用エラストマーの世界市場規模は?
→MarketsandMarkets社は2024年の医療用エラストマーの世界市場規模を95.7億米ドルと推定しています。
・医療用エラストマーの世界市場予測は?
→MarketsandMarkets社は2029年の医療用エラストマーの世界市場規模を142.7億米ドルと予測しています。
・医療用エラストマー市場の成長率は?
→MarketsandMarkets社は医療用エラストマーの世界市場が2024年~2029年に年平均8.3%成長すると予測しています。
・世界の医療用エラストマー市場における主要企業は?
→MarketsandMarkets社は「BASF (Germany), Dow (US), Celanese Corporation (US), Eastman Chemical Company (US), Syensqo (Belgium), Mitsubishi Chemical Group Corporation (Japan), Kuraray Co., Ltd. (Japan), ExxonMobil (US), Momentive Performance Materials (US), Envalior (Germany) and Zeon Corporation (Japan)など ...」をグローバル医療用エラストマー市場の主要企業として認識しています。
※上記FAQの市場規模、市場予測、成長率、主要企業に関する情報は本レポートの概要を作成した時点での情報であり、納品レポートの情報と少し異なる場合があります。











